In 2006, the United States (US) Food and Drug Administration published advisory highlighting concerns for late drug-eluting stent thrombosis; its impact on US bare-metal stent (BMS) utilization is unknown. We examined rates of BMS use among Medicare patients at 946 US hospitals in the CathPCI Registry who underwent percutaneous coronary intervention (PCI) during 3 periods: (1) 2004 to 2006 preadvisory (n = 166,458); (2) 2007 to 2008 postadvisory (n = 216,318); and (3) 2012 to 2014 contemporary (n = 827,948). We examined predicted risks of target vessel revascularization and bleeding among BMS recipients by period. We compared 1-year repeat revascularization and death/myocardial infarction risks among BMS recipients immediately preadvisory and postadvisory. BMS were used in 15.8% of preadvisory, 40.9% of postadvisory, and 20.0% of contemporary PCI procedures. Although 19.5% of preadvisory BMS patients had a predicted target vessel revascularization risk ≥15%/year, this decreased to 16.7% postadvisory (p <0.001), and increased back to 18.7% among contemporary BMS recipients (p <0.001). In contrast, 12.3% of preadvisory BMS recipients had a predicted bleeding risk ≥5%/year, compared with 14.6% postadvisory (p <0.001), and 18.2% in contemporary BMS recipients (p <0.001). Postadvisory BMS recipients had a lower risk of repeat revascularization (12.8% vs 14.6%, adjusted hazard ratio 0.87, 95% CI 0.84 to 0.90) but no difference in the composite risk of death/myocardial infarction (15.9% vs 15.9%, adjusted hazard ratio 0.97, 95% CI 0.93 to 1.00). In conclusion, a surge in BMS use after the advisory was not associated with an increased risk of repeat revascularization or adverse outcomes in BMS-treated patients. One in 5 contemporary PCI procedures still involve BMS implantation.
Drug-eluting stents (DESs) rapidly replaced bare-metal stents (BMSs) when DES implantation showed lower rates of in-stent restenosis and was approved by the US Food and Drug Administration (FDA) in 2003. Yet follow-up studies raised concerns that DES might be associated with an increased risk of late stent thrombosis. The FDA issued an advisory statement in 2006 that recognized the risk of late stent thrombosis, particularly when used in an off-label manner. Following this statement, rates of DES implantation decreased dramatically. Little is known about how patient selection for BMS use changed after the FDA advisory, and whether such patterns of device selection affected long-term outcomes after BMS implantation, including the risks of repeat revascularization, death, or myocardial infarction (MI). Using data from the CathPCI Registry, we examined temporal trends in BMS utilization before and after the FDA advisory and target vessel revascularization (TVR) and bleeding risk among 3 cohorts of patients. Furthermore, we compared longitudinal outcomes post-BMS implantation between the immediate preadvisory and postadvisory patients.
Methods
The CathPCI Registry is an initiative of the American College of Cardiology Foundation. The registry collects clinical data for consecutive percutaneous coronary intervention (PCI) procedures performed at more than 1,000 cardiac catheterization facilities in the United States. The linkage between the CathPCI Registry and Medicare inpatient claims files permits assessment of longitudinal outcomes beyond the initial PCI hospitalization. Briefly, International Classification of Diseases, Ninth Revision -9 procedure codes were used to identify index PCI procedures in the Medicare files which were then linked to CathPCI Registry records using a combination of indirect identifiers.
We compared 3 cohorts of Medicare-insured patients in the CathPCI Registry who underwent PCI during: (1) the preadvisory period from October 1, 2004, to September 31, 2006; (2) the immediate postadvisory period from January 1, 2007, to December 31, 2008; and (3) a more contemporary period from January 1, 2012, and December 31, 2014. As the FDA statement was issued in the final quarter of 2006, this quarter was excluded from the analysis. Patients aged ≥65 years who underwent their first PCI during the respective periods were included (n = 442,734). Patients were excluded if they received coronary artery bypass graft surgery during the index admission (n = 6,192), the index PCI was performed on a previously treated lesion (n = 30,730), or the index PCI stent type was unknown (n = 23,036).
Stent type (DES vs BMS) was reported on the CathPCI Registry data collection form; patients who received both BMS and DES were counted as BMS recipients. We examined the predicted risks of TVR and bleeding for patients who underwent PCI with BMS implantation in each period. The predicted risks of TVR and major bleeding were calculated for each patient using previously validated risk models.
Observed outcomes of readmission for repeat revascularization, composite of death, or readmission for MI by 1 year after PCI, death, and readmission for MI, were compared between the preadvisory and postadvisory periods. Longitudinal Medicare data are not available for the 2012 to 2014 contemporary population currently. Death was ascertained from Medicare denominator files, and the other outcomes were ascertained using the following International Classification of Diseases, Ninth Revision -9 diagnosis and procedure codes: 410.x1 for MI; 00.66, 36.01 to 36.02, 36.02 to 36.07, and 36.09 for PCI; and 36.10 to 36.19 for coronary artery bypass graft.
Baseline clinical and angiographic characteristics were compared among BMS recipients in the 3 periods referenced to the preadvisory period. Categorical variables were compared using the chi-square test and continuous variables using the Wilcoxon rank-sum tests. The cumulative incidence rates for time-to-event outcomes were estimated and differences were tested using Gray’s method. Cox proportional hazards modeling was used to examine unadjusted and adjusted associations between preadvisory versus postadvisory status and outcomes at 1 year in BMS and DES recipients, as well as the overall cohort of patients with PCI. Multivariable analyses adjusted for variables in the CathPCI Registry long-term mortality risk model. Secondary analyses compared preadvisory and postadvisory outcomes stratified by age < or ≥75 years.
Results
Among PCI procedures performed at 1,581 hospitals in the United States, BMS were implanted in 15.9% of preadvisory PCI procedures (26,590 of 166,458), compared with 40.9% of immediate postadvisory PCI procedures (88,426 of 216,318) and 20.0% of contemporary PCI procedures (165,316 of 827,948) in the 2012 to 2014 cohort. Characteristics of overall PCI, BMS, and DES recipients are described in Table 1 .
| Variable | All Recipients (n = 1,210,724) | Overall DES Recipients (n = 930,392) | Overall BMS Recipients (n = 280,332) |
|---|---|---|---|
| Age (years) | 74 (69,79) | 73 (69,79) | 75 (70,81) |
| Men | 742,025 (61.3%) | 572,298 (61.5%) | 169,727 (60.5%) |
| White | 338,303 (88.5%) | 236,535 (88.5%) | 101,768 (88.6%) |
| Prior PCI | 389,060 (32.1%) | 319,260 (34.3%) | 69,800 (24.9%) |
| Prior Coronary Bypass | 260,648 (21.5%) | 199,845 (21.5%) | 60,803 (21.7%) |
| Prior Myocardial Infarction | 321,933 (26.6%) | 249,767 (26.9%) | 72,166 (25.7%) |
| Prior Heart Failure | 182,021 (15.0%) | 131,971 (14.2%) | 50,050 (17.9%) |
| Peripheral Arterial Disease | 181,736 (15.0%) | 135,168 (14.5%) | 46,568 (16.6%) |
| Cardiovascular Disease | 195,332 (16.1%) | 144,027 (15.5%) | 51,305 (18.3%) |
| BMI (kg/m2) | 28.0 (24.9,31.9) | 28.2 (25.1,32.0) | 27.6 (24.4,31.5) |
| Diabetes mellitus | 446,706 (36.9%) | 349,203 (37.5%) | 97,503 (34.8%) |
| Dyslipidemia | 1029997 (85.1%) | 794,544 (85.4%) | 235,453 (84.0%) |
| GFR (mL/min) | 66.8 (52.2,84.7) | 68.3 (52.8,85.4) | 64.0 (49.6,79.2) |
| New York Heart Association Class | |||
| I | 128,957 (25.9%) | 94,011 (26.7%) | 34,946 (23.9%) |
| II | 97,322 (19.5%) | 97,322 (19.5%) | 26,653 (18.2%) |
| III | 116,742 (23.4%) | 83,320 (23.7%) | 33,422 (22.8%) |
| IV | 82,344 (16.5%) | 52,956 (15.0%) | 29,388 (20.1%) |
| Indication | |||
| Stable angina pectoris | 186,863 (15.4%) | 153,675 (16.5%) | 33,188 (11.8%) |
| Unstable angina pectoris | 464,258 (38.4%) | 379,347 (40.8%) | 84,911 (30.3%) |
| Non-ST elevation Myocardial Infarction | 251,107 (20.7%) | 184,527 (19.8%) | 66,580 (23.8%) |
| ST elevation Myocardial Infarction | 153,218 (12.7%) | 98,361 (10.6%) | 54,857 (19.6%) |
| Angiographic/Percutaneous Coronary Intervention Characteristics | |||
| Pre-procedure TIMI flow | |||
| 0 | 135,841 (11.3%) | 92,912 (10.0%) | 42,929 (15.4%) |
| 1 | 105,646 (8.8%) | 79,810 (8.6%) | 25,836 (9.3%) |
| 2 | 244,623 (20.3%) | 188,969 (20.4%) | 55,654 (19.9%) |
| 3 | 719,116 (59.7%) | 564,435 (60.9%) | 154,681 (55.4%) |
| Stent diameter (mm) | 2.8 (2.5,3.0) | 2.5 (2.5,3.0) | 3.0 (2.5,3.5) |
| Total stent (mm) | 35.0 (24.0,51.0) | 35.0 (24.0,52.0) | 33.0 (23.0,48.0) |
| ACC/AHA class C lesion | 578,398 (47.8%) | 450,043 (48.4%) | 128,355 (45.8%) |
| Bifurcation lesion | 130,983 (10.8%) | 105,755 (11.4%) | 25,228 (9.0%) |
| Multi-vessel Percutaneous Coronary intervention | 184,526 (15.2%) | 145,770 (15.7%) | 38,756 (13.8%) |
| Predicted risks | |||
| Target vessel revascularization risk † | 11.39% (9.52,14.02) | 11.52% (9.52,14.04) | 11.24% (9.37,13.88) |
| High risk for TVR (≥15%) | 236,138 (19.5%) | 185,333 (19.9%) | 50,805 (18.1%) |
| Bleeding risk ‡ | 1.90% (1.18,3.20) | 1.81% (1.14,2.99) | 2.27% (1.36,3.95) |
| High bleeding risk (≥5%) | 126,326 (10.4%) | 79,960 (8.6%) | 46,366 (16.5%) |
∗ p Value for all variables were <0.001 when overall BMS recipients were compared with overall DES recipients.
† TVR risk: model predicted annual risk of target vessel revascularization.
Characteristics of BMS recipients in each time period are listed in Table 2 . Although 19.5% of preadvisory BMS patients had a predicted TVR risk ≥15%/year, this decreased to 16.7% postadvisory (p <0.001), increasing back to 18.7% among contemporary BMS recipients (p <0.001; Table 2 ). Later cohorts had higher proportions of patients predicted to be at high risk for bleeding (≥5% risk): 12.3% of preadvisory BMS recipients versus 14.6% of postadvisory BMS recipients versus 18.2% of contemporary BMS recipients ( Table 2 ).
| Variable | Pre-Advisory 2004-2006 (n = 26,590) | Post-Advisory 2007-2008 (n = 88,426) | Contemporary 2012-2014 (n = 165,316) | p-value (Pre- vs. Post- advisory) | p-value (Pre-advisory vs. Contemporary) |
|---|---|---|---|---|---|
| Age (years) | 75.0 (70.0-80.0) | 75.0 (70.0-81.0) | 75.0 (70.0-82.0) | <0.001 | <0.001 |
| Men | 15,976 (60.1%) | 51,667 (58.4%) | 102,084 (61.8%) | <0.001 | <0.001 |
| White | 24,141 (90.9%) | 77,627 (87.9%) | 740, 073 (89.3%) | <0.001 | |
| Prior Percutaneous Coronary Intervention | 6,510 (24.5%) | 19.057 (21.6%) | 44,233 (26.8%) | <0.001 | <0.001 |
| Prior Coronary Bypass | 7,670 (28.8%) | 20,070 (22.7%) | 33,065 (20.0%) | <0.001 | <0.001 |
| Prior Myocardial Infarction | 7,657 (28.8%) | 21,147 (23.9%) | 43,362 (26.2%) | <0.001 | <0.001 |
| Prior Heart Failure | 4,263 (16.0%) | 13,729 (15.5%) | 32,058 (19.4%) | 0.05 | <0.001 |
| Peripheral Arterial Disease | 4,659 (17.5%) | 14,361 (16.2%) | 27,548 (16.7%) | <0.001 | <0.001 |
| Cardiovascular Disease | 4,818 (18.1%) | 15,172 (17.2%) | 31,315 (19.0%) | <0.001 | 0.001 |
| Diabetes mellitus | 8,807 (33.1%) | 28,749 (32.5%) | 59,947 (36.3%) | 0.06 | <0.001 |
| GFR (mL/min) | 63.1 (49.0-77.0) | 63.3 (49.0-77.2) | 64.7 (49.9,84.7) | 0.16 | <0.001 |
| New York Heart Association class at presentation | |||||
| I | 8,3671 (31.5%) | 24,220 (27.4%) | 2,355 (7.5%) | <0.001 | <0.001 |
| II | 5,190 (19.5%) | 19,108 (21.6%) | 7,520 (24.0%) | <0.001 | <0.001 |
| III | 7,275 (27.4%) | 23,792 (26.9%) | 11,920 (38.0%) | 0.15 | <0.001 |
| IV | 5,751 (21.6%) | 21,282 (24.1%) | 9,595 (30.6%) | <0.001 | <0.001 |
| Indication for Percutaneous Coronary Intervention | |||||
| Stable angina pectoris | 3,792 (14.3%) | 10.981 (12.4%) | 18,415 (11.1%) | <0.001 | <0.001 |
| Unstable angina pectoris | 8.199 (30.8%) | 24,608 (27.8%) | 52,104 (31.5%) | <0.001 | 0.03 |
| Non-ST elevation Myocardial Infarction | 4,971 (18.7%) | 18,910 (21.4%) | 42,699 (25.8%) | <0.001 | <0.001 |
| ST elevation Myocardial Infarction | 3,907 (14.7%) | 15,877 (18.0%) | 35,073 (21.2%) | <0.001 | <0.001 |
| Angiographic/Percutaneous Coronary Intervention characteristics | |||||
| Pre-procedure Thrombolysis in Myocardial Infarction (TIMI) flow | |||||
| TIMI 0 | 3,094 (11.7%) | 12,377 (14.1%) | 27,465 (16.7%) | <0.001 | <0.001 |
| TIMI 1 | 2,620 (9.9%) | 9,424 (10.7%) | 13,783 (8.4%) | <0.001 | <0.001 |
| TIMI 2 | 5,176 (19.6%) | 17,700 (20.1%) | 32,793 (19.9%) | 0.08 | 0.38 |
| TIMI 3 | 15,494 (58.7%) | 48,481 (55.1%) | 90,695 (55.1%) | <0.001 | <0.001 |
| Stent diameter (mm) | 3.0 (2.5,4.0) | 3.0 (2.5,3.5) | 2.5 (2.5,3.0) | 0.42 | <0.001 |
| Total stent length (mm) | 32.0 (21.0-47.0) | 32.0 (21.0-46.0) | 35.0 (24.0,49.0) | 0.03 | <0.001 |
| ACC/AHA Class C lesion | 11,038 (41.5%) | 35,433 (40.1%) | 81,880 (49.6%) | <0.001 | <0.001 |
| Bifurcation lesion | 2,044 (7.7%) | 7,232 (8.2%) | 15,938 (9.7%) | 0.01 | <0.001 |
| Multi-vessel Percutaneous Coronary Intervention | 5,055 (19.0%) | 12,823 (14.5%) | 20,878 (12.6%) | <0.001 | <0.001 |
| Predicted risk | |||||
| Target vessel revascularization risk ∗ | 11.2 (9.1%, 41.0%) | 10.9 (8.9%, 13.5%) | 11.4 (9.5%, 14.0%) | <0.001 | <0.001 |
| High risk for TVR (≥15%) | 5,195 (19.5%) | 14,753 (16.7%) | 30,857 (18.7%) | <0.001 | <0.001 |
| Bleeding risk † | 2.0 (1.24%, 3.35%) | 2.2 (1.3, 3.7) | 2.4 (1.4%, 4.2%) | <0.001 | <0.001 |
| High bleeding risk (≥5%) | 3,258 (12.3%) | 12,945 (14.6%) | 30,163 (18.2%) | <0.001 | <0.001 |
∗ TVR risk: model predicted annual risk of target vessel revascularization.
The use of evidence-based medications for both BMS and DES changed significantly from the preadvisory period to the contemporary period ( Table 3 ). In particular, the use of β blockers and statins increased significantly from the preadvisory period to the contemporary period. Clopidogrel use rates downtrended over time after FDA approval of prasugrel and ticagrelor as antiplatelet options.
| Variable | Pre-Advisory 2004-2006 (n = 166,458) | Post-Advisory 2007-2008 (n = 216,318) | Contemporary 2012-2014 (n = 827,948) | p-value (Pre- vs. Post- advisory) | p-value (Pre-advisory vs. Contemporary) |
|---|---|---|---|---|---|
| Periprocedural P2Y12 Use | 122,905 (74.0%) | 171,714 (79.6%) | 747,860 (90.5%) | <0.001 | <0.001 |
| Periprocedural Clopidogrel Use | 122,429 (73.9) | 171,166 (79.6) | 574,857 (69.7) | <0.001 | <0.001 |
| Discharge Medications | |||||
| ACE Inhibitor | 76,688 (48.1%) | 101,761 (49.3%) | 373,976 (48.9%) | <0.001 | <0.001 |
| ARBs | 22,324 (14.0%) | 34,369 (16.5%) | 145,669 (18.7%) | <0.001 | <0.001 |
| Aspirin | 153,786 (95.2%) | 201,710 (96.2%) | 775,458 (98.1%) | <0.001 | <0.001 |
| Beta Blocker | 124,823 (78.2%) | 168,362 (81.5%) | 669,646 (86.8%) | <0.001 | <0.001 |
| Statin | 129,811 (80.2%) | 175,089 (83.7%) | 721,189 (92.9%) | <0.001 | <0.001 |
| Clopidogrel | 157,637 (96.9%) | 206,585 (97.9%) | 621,506 (78.0%) | <0.001 | <0.001 |
∗ All abbreviations can be found in Table 1 .
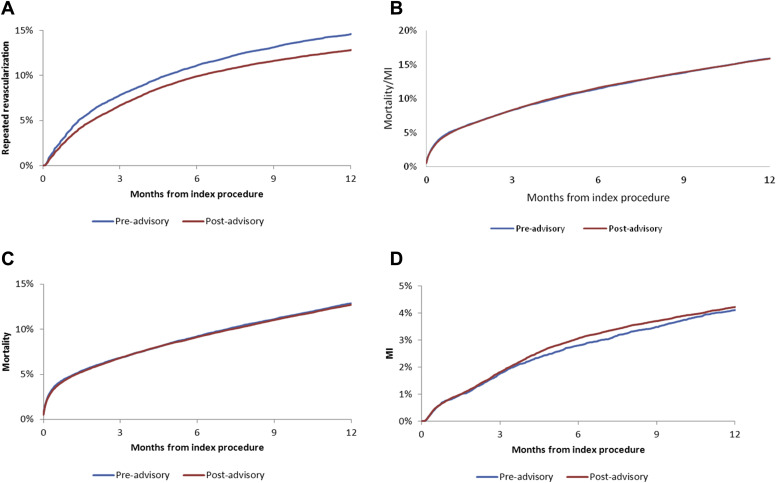
We compared 1-year outcomes of repeat revascularization, and the composite of death/MI, death, and readmission for MI, between BMS recipients in the preadvisory (2004 to 2006) and postadvisory (2007 to 2008) periods. Unadjusted cumulative incidence curves are shown in Figure 1 . Compared with the preadvisory period, postadvisory BMS recipients were associated with having a lower risk of repeat revascularization, but there was no significant difference in the unadjusted risk of death or MI at 1 year. After multivariable adjustment, postadvisory BMS recipients remained associated with a lower risk of repeat revascularization by 1 year compared with preadvisory BMS recipients (adjusted hazard ratio 0.87, 95% CI 0.84 to 0.90). There was no significant difference in the adjusted risks of composite death/MI or MI alone between the preadvisory cohort and the immediate postadvisory cohort at 1 year ( Table 4 ). Compared with preadvisory BMS recipients, postadvisory BMS recipients had a modestly lower adjusted risk of 1-year mortality.
| Term | BMS Recipients Adjusted | DES Recipients Adjusted | All PCI Patients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Repeat revascularization | |||||||||
| 1 year | 0.87 | 0.84-0.90 | <0.001 | 0.90 | 0.87-0.92 | <0.001 | 0.89 | 0.87-0.91 | <0.001 |
| Composite: Death/Myocardial Infarction | |||||||||
| 1 year | 0.97 | 0.93-1.00 | 0.07 | 0.98 | 0.95-1.00 | 0.081 | 0.97 | 0.95-0.99 | <0.004 |
| Death | |||||||||
| 1 year | 0.95 | 0.91-0.98 | 0.01 | 0.98 | 0.95-1.01 | 0.12 | 0.96 | 0.94-0.98 | <0.001 |
| Myocardial Infarction | |||||||||
| 1 year | 1.06 | 0.98-1.13 | 0.13 | 0.97 | 0.92-1.02 | 0.24 | 1.00 | 0.96-1.04 | 0.92 |
∗ Immediate postadvisory period described in the table above uses 2006 to 2008 data.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


