Treatment of Splanchnic and Renal Artery Aneurysms
James C. Stanley
Gilbert R. Upchurch Jr.
Peter K. Henke
The surgical treatment of the many types of splanchnic and renal artery aneurysms depends on a recognition of the disease’s underlying pathology, its clinical relevance, and the various therapeutic options. The individual aneurysms and their management deserve separate discussion.
Splenic Artery Aneurysms
Splenic artery aneurysms account for about 60% of all splanchnic aneurysms. Women are affected four times more often than men. Splenic artery aneurysms most often are saccular, usually occur at bifurcations, and are multiple in approximately 20% of patients.
Three conditions contribute to the development of these aneurysms. The first is systemic arterial dysplasia, with 4% of patients having documented renal artery fibrodysplasia also having splenic artery aneurysms. Portal hypertension is a second factor, with 7% of these patients exhibiting these aneurysms. Vessel wall changes that cause the increased splenic artery diameters, which are known to occur in portal hypertension, may account for the aneurysmal changes as well. A third factor relates to the vascular effects of repeated pregnancy. Approximately 45% of women with splenic artery aneurysms are grand multiparas, having completed six or more pregnancies. Aneurysm formation in these patients may be due to the hormonal effects on elastic tissue and increased splenic arteriovenous shunting occurring during pregnancy. Although many splenic artery aneurysms exhibit calcific arteriosclerosis, this is considered a secondary event rather than the cause of most lesions. Additional causes of these aneurysms include trauma, as well as a variety of inflammatory diseases, particularly pancreatitis with associated pseudocyst formation.
Splenic artery aneurysms are usually asymptomatic. Vague left upper quadrant or epigastric discomfort is a nonspecific symptom occasionally attributed to these lesions. Roentgenographic demonstration of left upper quadrant, curvilinear, signet ring-like calcification may suggest the presence of a splenic artery aneurysm, but most of the aneurysms are recognized as incidental findings during imaging studies, including arteriography, computed tomography (CT), and magnetic resonance angiography (MRA) for unrelated diseases.
Rupture of asymptomatic splenic artery aneurysms occurs in less than 2% of instances. The mortality of rupture in non-pregnant patients is less than 25%. Rupture has been reported in more than 90% of aneurysms recognized during pregnancy, with maternal mortality approaching 75% and fetal mortality exceeding 95%. It is likely that many unruptured splenic artery aneurysms exist during pregnancy and go unrecognized clinically. Rupture usually presents with hemorrhage into the lesser sac, with distant symptoms following as blood escapes through the foramen of Winslow. Lesser sac tamponade may postpone catastrophic intraperitoneal bleeding, which accounts for the so-called double rupture presentation of many aneurysms.
Treatment of splenic artery aneurysms is justified in pregnant patients, women of childbearing age, and all patients with symptomatic aneurysms. Elective operations for asymptomatic splenic artery aneurysms that measure 2 cm or greater in diameter are appropriate when operative mortality is less than 0.5%. If surgical therapy entails a high risk, transcatheter embolization of the aneurysm represents the favored alternative form of management.
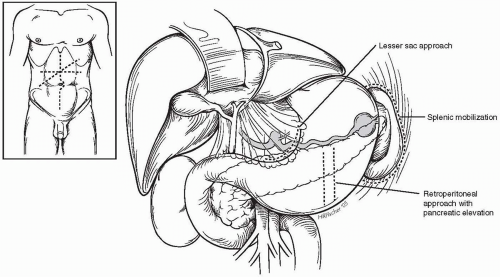
Splenic artery aneurysms may be approached through one of several anterior abdominal wall incisions. Extended right subcostal, transverse epigastric, and vertical midline incisions are all suitable, with the specific exposure selected depending on the patient’s disease process and the planned procedure. Proximal aneurysms are best exposed through the lesser sac. Aneurysms in the midsplenic artery may be exposed with a retroperitoneal approach after pancreatic mobilization and elevation. Aneurysms located in the distal artery or splenic hilus are exposed with splenic mobilization (Fig. 23-1). Laparoscopic management of these lesions may provide a less hazardous alternative to conventional operation.
Proximal and Midsplenic Artery Aneurysmectomy or Exclusion
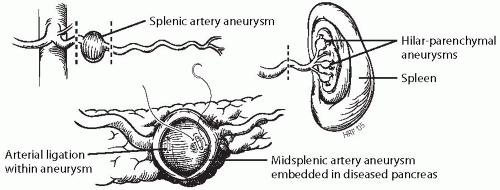
Proximal splenic artery aneurysms are usually treated by aneurysmectomy or exclusion, with splenic artery ligation without arterial reconstruction (Fig. 23-2). The proximal splenic artery is easily exposed by dividing the gastrohepatic ligament along the lesser curve of the stomach. Entering and exiting vessels are ligated, and the aneurysm is excised if it is not embedded within pancreatic tissue. In the latter situation, the aneurysm is not removed, but it must be opened to assure that all branches are ligated.
Certain splenic artery aneurysms, especially false aneurysms associated with pancreatic inflammatory disease, may not be easily excised. False aneurysms occurring as a consequence of pancreatic pseudocyst erosions into the splenic artery, when responsible for active hemorrhage, are treated best by arterial ligation from within the aneurysm. Monofilament suture should be used in these instances. Internal or external drainage of a pseudocyst, if present, may be necessary after arterial ligation has been accomplished. Distal pancreatectomy, including the diseased artery, is often preferred when treating false aneurysms in patients who can tolerate the procedure.
Hilar and Parenchymal Splenic Artery Aneurysmectomy or Exclusion
Surgical therapy for most aneurysms within the hilus or substance of the spleen historically has been splenectomy. Standard surgical technique has usually been followed in these instances. However, splenic preservation to maintain host resistance with simple suture obliteration of distal aneurysms is preferable to splenectomy, even though segmental splenic infarction may occur.
Splenic Artery Aneurysm Endovascular Intervention
Percutaneous catheter-based treatment of splenic artery aneurysms is being used increasingly as the primary mode of treatment. Percutaneous transcatheter embolization of splenic artery aneurysms, especially in high-risk patients, such as in portal hypertensives, is the preferred alternative to open operative intervention in these patients. Stent-graft exclusion of splenic artery aneurysms has recently been shown to be feasible technically. Careful follow up of endovascular-treated patients is mandatory. Splenic infarction and late rupture may occur. Nondurable obliteration of the aneurysm and coil migration and erosion into the adjacent viscera are additional concerns that must be addressed in follow-up studies.
Hepatic Artery Aneurysms
Aneurysms of the hepatic artery, representing nearly 20% of all splanchnic artery aneurysms, are often life threatening. Medial degeneration, trauma, and infection account for 24%, 22%, and 10% of hepatic aneurysms, respectively. Arteriosclerosis, present in 32% of these aneurysms, is considered a secondary, not a causative, process.
A surprising observation was that 17% of these aneurysms encountered in recent times occurred in orthotopic liver transplantation patients. Women are affected twice as often as men. Excluding traumatic lesions, most hepatic artery aneurysms are encountered in patients older than 50 years of age.
A surprising observation was that 17% of these aneurysms encountered in recent times occurred in orthotopic liver transplantation patients. Women are affected twice as often as men. Excluding traumatic lesions, most hepatic artery aneurysms are encountered in patients older than 50 years of age.
Hepatic artery aneurysms are extrahepatic in 80% of cases, with 20% being intrahepatic. Generally, lesions that exceed 2 cm in diameter are saccular, and smaller aneurysms are fusiform. In a review of 163 aneurysms in which the specific site of arterial involvement was mentioned, 63% arose from the common hepatic artery, 28% in the right hepatic artery, 5% in the left hepatic artery, and 4% in both left and right hepatic arteries. Excluding microaneurysms associated with systemic arteritis, hepatic artery aneurysms are usually solitary.
Symptomatic intact aneurysms often produce right upper quadrant or epigastric pain, frequently ascribed to chronic cholecystitis. Severe pain may accompany acute aneurysmal expansion and be confused with pancreatitis. Rupture occurs in less than 20% of cases. The mortality of 35% from aneurysmal rupture has not changed during recent years. Rupture of hepatic artery aneurysms occurs with equal frequency into the peritoneal cavity and hepatobiliary tree. Rupture into the latter results in the hematobilia, manifest by biliary colic, periodic hematemesis, and jaundice. Erosion of aneurysms into the stomach, duodenum, and pancreatic duct occurs uncommonly. Extrahepatic rupture, usually of inflammatory aneurysms, frequently results in exsanguinating intraperitoneal hemorrhage.
Pre-operative diagnosis of hepatic artery aneurysms may be difficult. Aneurysmal calcifications are occasionally evident on abdominal roentgenograms. Displacement or compression of adjacent gastrointestinal structures seen during barium contrast studies or cholecystocholangiography may suggest the presence of these aneurysms. The more routine use of arteriography, CT, and MRA has resulted in more common recognition of these aneurysms. All hepatic artery aneurysms should be treated surgically unless inordinate operative risks are present.
Hepatic artery aneurysms are approached through an upper abdominal transverse, extended right subcostal, or vertical midline incision. The common and proper hepatic arteries are easily accessible through the lesser space. Initial palpation of aneurysms within the hepatoduodenal ligament often allows the surgeon to assess the relationship of an aneurysm to the common bile duct and portal vein, something that may prove difficult once dissection has begun. Proximal proper hepatic artery lesions should be dissected cautiously, with particular attention directed to the gastroduodenal artery and its pancreaticoduodenal branch, which often overlie and cross anterior to the common bile duct. Distal proper hepatic artery aneurysms near the hilus of the liver must also be dissected with great care to avoid bile duct injuries.
Hepatic Artery Aneurysm Ligation
Common hepatic artery aneurysms can occasionally be treated successfully by aneurysm exclusion without reconstruction of the involved vessel. The extensive foregut collateral circulation through the gastroduodenal and right gastric arteries usually ensures adequate blood flow to the liver. If liver blood appears compromised after temporary hepatic artery occlusion, then reconstruction of the diseased vessel must be pursued.
Hepatic Artery Aneurysmectomy and Primary Closure
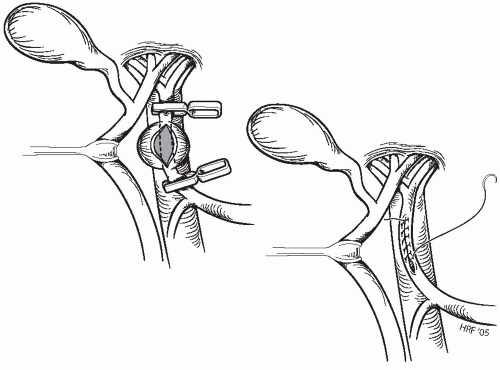
Aneurysmorrhaphy is appropriate in certain instances of saccular aneurysms, usually those associated with penetrating trauma (Fig. 23-3). Upon completing dissection of the hepatic artery proximal and distal to the aneurysm, microvascular clamps, developing tensions of 30 to 70 g, are used to occlude the hepatic vessels. After aneurysmectomy, a primary closure is performed using a fine monofilament cardiovascular suture in a continuous manner. Placement of the initial stitch at the distal apex of the arterial defect is critical in lessening the chance for narrowing of the vessel as the repair commences.
Hepatic Artery Aneurysmectomy and Interposition Graft Repair
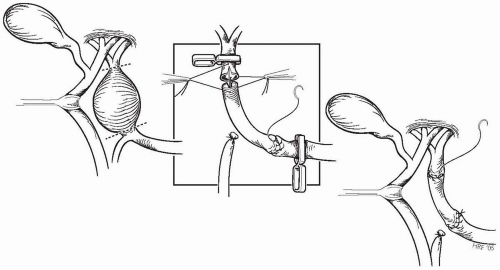
Fusiform and large saccular aneurysms of the proper hepatic artery are best treated by aneurysmectomy and formal reconstruction of the vessel. Interposition grafting is preferred (Fig. 23-4), with reversed autogenous saphenous vein being favored over prosthetic conduits. Vein grafts are carefully procured, gently handled, and flushed with heparinized blood before implantation. They are not distended with irrigation solutions, and disturbance of adventitial tissues is kept to a minimum as the graft is prepared.
Careful dissection and isolation of the aneurysm is performed, with ligation and transection of the gastroduodenal and
pancreaticoduodenal vessels, if they are involved with the aneurysm. Digital control of entering and exiting vessels and early entrance into the aneurysm, with additional control by the use of balloon catheters or rigid dilators from within, may be appropriate for large lesions and in those instances in which dissection of the artery from the surrounding biliary and venous structures might prove hazardous. Anastomoses with spatulation of the vein and artery are carried out in a standard manner.
pancreaticoduodenal vessels, if they are involved with the aneurysm. Digital control of entering and exiting vessels and early entrance into the aneurysm, with additional control by the use of balloon catheters or rigid dilators from within, may be appropriate for large lesions and in those instances in which dissection of the artery from the surrounding biliary and venous structures might prove hazardous. Anastomoses with spatulation of the vein and artery are carried out in a standard manner.
Hepatic Artery Aneurysmectomy and Aortohepatic Bypass
Aortohepatic bypass (Fig. 23-5) is preferable to other reconstructions if the common hepatic artery is not a suitable inflow vessel for an interposition graft. Following dissection of the aneurysm, an extended Kocher maneuver is performed to expose the aorta and inferior vena cava. A segment of saphenous vein, adequate in length for the aortohepatic bypass, is carefully procured. The patient is subsequently anticoagulated with heparin. An anterolateral aortotomy is made approximately twice the diameter of the vein. The reversed saphenous vein is anastomosed to the aorta using a continuous monofilament suture.
The distal hepatic artery beyond the aneurysm is occluded with a microvascular clamp, the proximal vessel is ligated, and then the aneurysm is excised. The hepatic artery is spatulated anteriorly and the vein graft is spatulated posteriorly. Two initial fine monofilament cardiovascular sutures are placed in the apex of the spatulation and the free border of the adjacent vessel to serve as stay stitches. The anastomosis is completed with a continuous suture, after which the clamps are released and antegrade flow to the liver is restored.
Hepatic Artery Aneurysm Endovascular Intervention
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree