Scott Kinlay, Deepak L. Bhatt
Treatment of Noncoronary Obstructive Vascular Disease
Peripheral vascular disease is a general term that includes pathologic processes affecting arteries, veins, and lymphatics (see also Chapter 58). This chapter focuses on catheter-based endovascular treatment of large and medium-sized arteries predominantly affected by atherosclerosis, as well as large vein obstruction secondary to chronic disease. Although peripheral artery disease (PAD) refers to lower limb arterial disease, it is sometimes used to describe arterial disease in the large and medium arteries of the upper limbs, cervical arteries, and aortomesenteric arteries. The incidence and prevalence of PAD increase with age and with other risk factors for atherosclerosis. Thus these two demographic forces are likely to increase PAD in industrialized and rapidly modernizing nations throughout the world where average age is increasing and risk factors are prevalent.
Increasing awareness of PAD, the impact that PAD has on cardiovascular risk and quality of life, and the rapid development of percutaneous techniques for revascularization continue to accelerate the number of endovascular procedures for PAD. Appropriate use of this expensive technology requires a clear understanding of the goals of medical and revascularization therapies.
Approach to the Patient with Peripheral Artery Disease
Chronic PAD is either asymptomatic or associated with symptoms such as claudication, critical limb ischemia, or embolic infarction of a distal organ (e.g., stroke). Asymptomatic disease is common. In the lower extremities, asymptomatic disease occurs in at least half and in as many as 80% of subjects with abnormal functional test results indicative of obstructive arterial disease (e.g., abnormal ankle-brachial index [ABI]). Even asymptomatic disease is a marker for elevated cardiovascular risk.1–4 Therefore a prime goal of therapy is intensive modification of atherosclerosis risk factors to reduce the risk for myocardial infarction and stroke, which are the most common causes of death in patients with PAD.3,5
Claudication classically refers to leg discomfort or pain related to exercise and relieved by rest, but it is also used to describe discomfort in the upper limbs caused by effort-related ischemia. Claudication affects function (the ability to walk or use a limb) and quality of life. Hence treatment of claudication aims to improve function and reduce discomfort at the maximum level of activity desired by a patient. The two most important lifestyle interventions for claudication are to stop cigarette smoking and to start a regular walking regimen. Together, these interventions reduce the mechanisms responsible for the progression of disease and favorably change arterial biology—for example, vasodilator function, muscle function, and angiogenesis.2,3,5 It is important to tell patients that the pain or discomfort associated with claudication is not harmful and that once this discomfort abates with rest, they should continue to push their activity again to improve endurance. Revascularization strategies aim to improve arterial blood flow in obstructed large and medium-sized arteries when noninvasive therapies fail. Catheter-based intervention, when indicated, should be deployed together with lifestyle and medical treatment.6
Critical limb ischemia refers to PAD with ischemic pain at rest or tissue loss (e.g., ulcer or gangrene).2,3 This scenario has clinical urgency because of near-term risk for limb jeopardy requiring major amputation. Major amputation in the lower limbs refers to amputation at or above the level of the ankle and requires a prosthesis for the patient to walk.3 It is disfiguring, and higher levels of amputation have greater impact on functional independence of the patient. In contrast, minor amputations (e.g., toe or transmetatarsal) usually have little impact on the patient’s ability to walk. Catheter-based therapies for critical limb ischemia aim to improve blood flow to heal ischemic tissue, to salvage the limb (prevent major amputation), or to enable a lower level of amputation that might have less impact on the patient’s ability to walk.
Cervical carotid, vertebral, and subclavian disease, although often asymptomatic, can lead to artery-to-artery embolism with transient ischemic attack and stroke. The risk for major stroke is high shortly after a symptomatic event but less so several months after an event or with asymptomatic disease.7 Mesenteric and renal artery disease affects the function of these organs. Chronic ischemia of the gut causes postprandial abdominal discomfort and food avoidance leading to weight loss, but it may progress to frank mesenteric infarction with a high mortality rate.8 Renal artery stenosis can precipitate hypertensive crises associated with pulmonary edema, hypertension resistant to treatment, and rapidly worsening renal dysfunction.9
Symptomatic disease that threatens a distal organ (e.g., critical limb ischemia, transient ischemic attack, or mesenteric angina) justifies a more aggressive approach because these manifestations entail the highest risk for functional loss and death without treatment. PAD associated with less threatening clinical scenarios (e.g., claudication) may allow a less aggressive approach with more time to implement lifestyle and medical therapies (Fig. 60-1). There is rarely justification for catheter-based or surgical revascularization of asymptomatic lower or upper limb PAD, mesenteric disease, or subclavian or vertebral artery disease. The value of revascularizing asymptomatic extracranial carotid disease, beyond medical therapy, is uncertain, although it is supported by recent guidelines involving patients at higher risk for stroke and low risk for periprocedural adverse events.7
Quality of Evidence Evaluating Endovascular Treatments
In contrast to coronary disease, only a few well-controlled studies have evaluated endovascular treatment of PAD and venous disease. Many studies are single arm, and most focus on patency (lack of restenosis) and repeated revascularization over a relatively short period. Although these endpoints provide information on the mechanisms likely to lead to improved control of symptoms, function, quality of life, and tissue preservation, they do not provide direct guidance on symptoms and function in patients with claudication or critical limb ischemia. Interventionalists should recognize the limitations in many studies and encourage future studies to address patient-oriented endpoints and adjudicated cardiovascular outcomes.10,11
Endovascular Technologies
Balloon Angioplasty
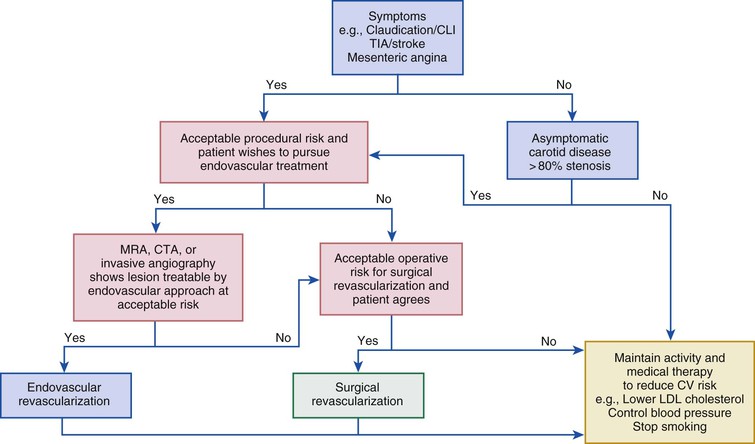
Balloon angioplasty remains the mainstay of endovascular intervention for PAD and venous disease (Fig. 60-2). Angioplasty remodels the artery by expansion and accommodates the atherosclerotic plaque to expand the vessel lumen. This procedure usually causes dissection of the plaque that may or may not impair blood flow. Angioplasty is limited in the short term by acute recoil of the artery and flow-limiting dissections, which may cause abrupt closure of the artery. In the intermediate time frame, overexuberant neointimal hyperplasia and negative remodeling of the artery may lead to symptomatic restenosis. Despite these limitations, balloon angioplasty can achieve durable results, particularly with shorter lesions, and is less likely than stenting to obstruct side branches associated with the lesion. Most operators use prolonged inflations (a minute or more).12 Both rapid-exchange and over-the-wire platforms are available, as well as short and long shaft lengths for lesions close or farther away from the access site.
Bare Metal Stents
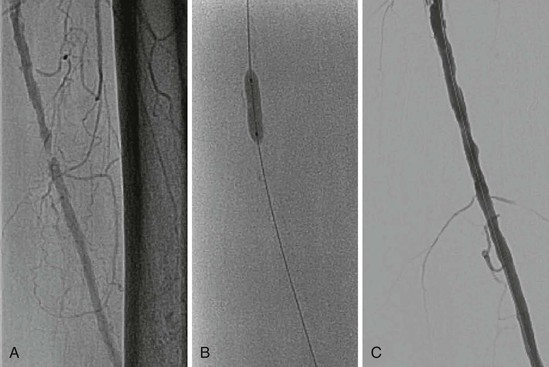
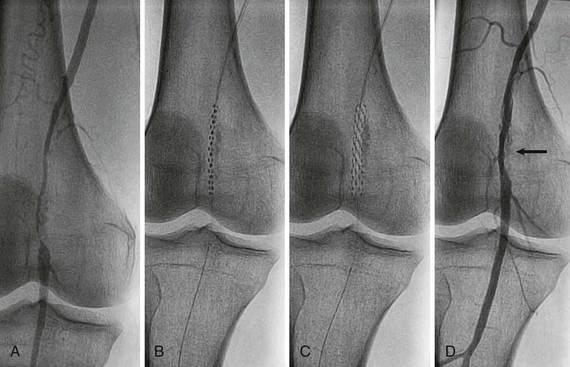
Bare metal stents come in two types—either balloon-expandable (Fig. 60-3) or self-expandable stents (Fig. 60-4). Stent implantation requires aspirin therapy and a thienopyridine (e.g., clopidogrel), although the evidence for dual antiplatelet therapy is derived largely by extrapolation from the coronary stent literature.
Balloon-expandable stents have greater radial strength and are less likely to move on deployment—which is important for ostial placement. Such stents can be crushed by external compression and are therefore avoided outside the torso. They are sometimes used to treat tibial disease, but only for critical limb ischemia, for which long-term patency may be less of an issue once tissue healing has occurred.
Self-expanding stents were originally made of stainless steel but are now more commonly made of nitinol.12 Nitinol stents re-expand on compression and are therefore used outside the torso, where external compression is more likely to occur. They may also be used in tortuous arteries, where they probably conform better than balloon-expandable stents do. Their lower radial strength, however, increases the risk for recoil. More recent designs are more durable and less likely to fracture.12,13 Nitinol stents cannot be overdilated if the stent is undersized for the artery, which may lead to stent malapposition or even embolization.
Drug-Eluting Peripheral Stents
Earlier attempts at coating peripheral self-expanding stents were initially associated with less restenosis in the short term but were unsuccessful in longer follow-up, partly because of inferior stent platforms prone to fracture. More durable stent designs12–14 and drug elution with everolimus15 or paclitaxel16 offer lower rates of restenosis, although the magnitude of long-term benefit remains under investigation. The duration of dual antiplatelet therapy required for these stents is also uncertain, but recent randomized trials have generally used 2 to 6 months of treatment with a thienopyridine.15,16
Covered Stents
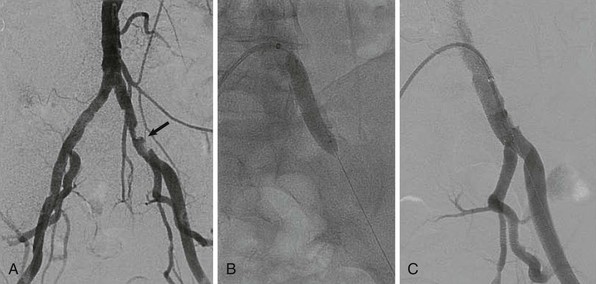
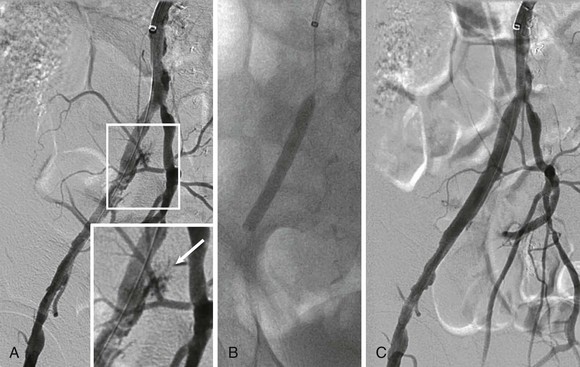
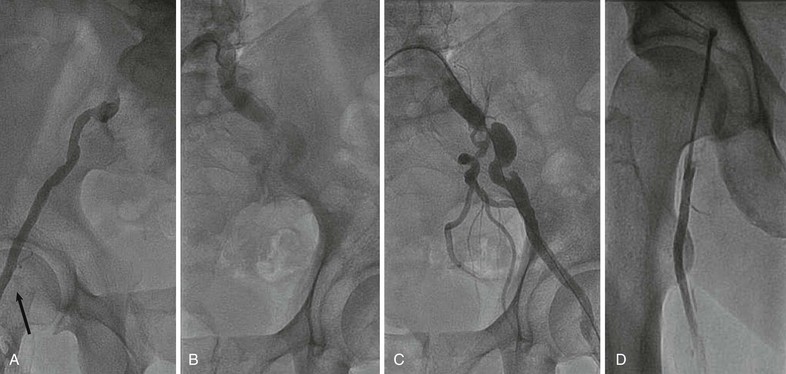
Stents covered with or sandwiching a polymer such as polytetrafluoroethylene (PTFE) have proved very useful for treating perforations related to endovascular treatment or excluding aneurysms (Fig. 60-5). Some trials have suggested that these designs may offer lower restenosis rates when treating femoral and iliac artery disease,17,18 but the benefit may reflect unusually high rates of restenosis in the control groups of these studies. Disadvantages of covered stents include unintentional occlusion of important branch vessels, concerns about the risk for late stent thrombosis, and whether restenosis was merely delayed rather than prevented.
Drug-Eluting Balloons
Balloons coated with antirestenosis agents (drug-eluting balloons) represent an exciting development over the last few years. This technology uses a non–stent-related method to deliver drugs such as paclitaxel into the arterial wall after conventional angioplasty treatments. Initial studies have shown great promise for this technology in preventing restenosis in the infrainguinal arteries (femoral and below).19–23 The risk for restenosis tends to lie between that of plain balloon angioplasty and drug-eluting stenting. Such a result is not surprising because this technology does not combat arterial recoil, an important contributor to late loss of lumen diameter. The durability of this treatment versus plain balloon angioplasty and bare metal or drug-eluting stents requires further definition. Similarly, the need for dual antiplatelet therapy is uncertain.
Thrombolysis and Thrombectomy
Catheter-directed thrombolysis is an important adjunctive therapy for arterial thrombosis, stent thrombosis, and occlusive thrombotic venous disease. Thrombolysis may be indicated for acute thrombosis with a threatened but viable extremity, but an immediately threatened limb (e.g., with sensory or early motor deficits) should be treated by surgical embolectomy, which offers more rapid reperfusion. Much of the experience with catheter-based thrombolysis is derived from its use for acute limb ischemia, venous thrombosis, or pulmonary embolism. It serves as an adjunctive treatment of semiacute manifestations such as peripheral stent thrombosis. Long-term results tend to be better when thrombolysis reveals an anatomic stenosis that probably precipitated the thrombosis and is treatable, for example, by repeated angioplasty.
Catheter-directed thrombolysis is more effective than intravenous thrombolysis only if an infusion catheter (with multiple infusion holes) is inserted into the thrombosed vessel. It is also less effective if given more than 14 days after thrombosis.3,4 Typically, the infusion is continued for 12 to 24 hours because treatment over a period of 48 hours is associated with depletion of circulating fibrinogen and a higher risk for major bleeding.24 Catheter-based thrombolysis with or without angioplasty or stenting also reduces the incidence of post-thrombotic syndrome in patients with proximal (iliac) deep venous thrombosis,25 and is used as adjunctive therapy for massive pulmonary emboli (see also Chapter 73).26,27
There is a small risk for fatal or major bleeding with any thrombolysis regimen. Absolute contraindications to thrombolysis3,26 include (1) a cerebrovascular event less than 2 months previously, (2) active bleeding, (3) gastrointestinal bleeding less than 10 days previously, and (4) neurosurgery (intracranial or spinal surgery) or trauma less than 3 months previously. Relative contraindications include (1) cardiopulmonary resuscitation less than 10 days previously, (2) nonvascular surgery or trauma less than 10 days previously, (3) uncontrolled hypertension (sustained systolic blood pressure >180 mm Hg or diastolic blood pressure >110 mm Hg), (4) puncture of a noncompressible vessel, (5) intracranial tumor, and (6) recent eye surgery.
Catheter aspiration thrombectomy uses catheters with a rapid-exchange port to direct the catheter to the thrombus and a large aspiration port to aspirate the catheter with a large syringe.3,4,26 These catheters can be used to aspirate smaller thrombi but are generally inadequate for a large burden of thrombus (e.g., long femoral stent thrombosis).
Mechanical thrombectomy includes a variety of devices that may include thrombolytic agents to help break up thrombus before suction by an aspiration catheter or catheters using the Venturi effect.3,4,26 Although mechanical thrombectomy is a more rapid treatment than catheter-directed thrombolysis, embolization can occlude the distal arterial bed and potentially lead to infarction and tissue loss.
Atherectomy and Other Treatments
Atherectomy devices, although conceptually attractive, have not proved better than angioplasty in direct comparisons in most arterial beds.12,28,29 Atherectomy is one of several niche tools and is most helpful in heavily calcified arteries to improve balloon and stent expansion or in regions where vessels are subjected to repetitive flexion or torsion, such as over joints, and where stents are avoided (because of kinking and increased fracture). In these settings, atherectomy may improve the distensibility of an artery to permit adequate expansion by balloon angioplasty without flow-limiting dissection. Drug-eluting balloons have renewed interest in this technology because they may reduce the contribution of excessive neointimal hyperplasia to restenosis. This strategy needs formal testing in clinical trials.
Coronary rotational atherectomy devices (Rotablator) are generally too small for the larger peripheral arteries, and it is uncertain how a large amount of plaque ablated from a long peripheral lesion would affect the downstream microcirculation (Fig. 60-6). Peripheral rotational atherectomy devices include the Jetstream, which has a 2.0-, 3.1-, and 3.5-mm cutting profile (Fig. 60-7), and the Diamondback, which uses an eccentric location to achieve a larger cutting arc.12,28,29 Directional atherectomy devices include the Silverhawk device (Fig. 60-8). All the peripheral devices have a tendency to embolize plaque into microvessels. Distal embolic protection devices may reduce this complication.
Cryoplasty involves the use of proprietary balloon and inflation technology to inflate the balloon with nitrous oxide, which chills on expansion to −10°C (Fig. 60-9). One pilot study suggested lower rates of restenosis in the femoral arteries when used with nitinol stents than when balloon angioplasty was performed,30 but longer-term outcomes are uncertain.12,28,29
Planning an Intervention
Vascular Imaging
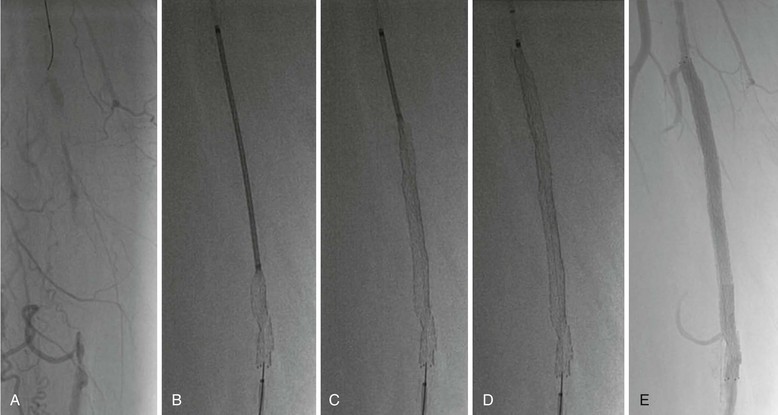
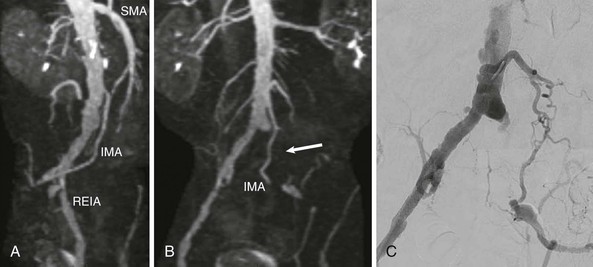
Vascular imaging is the first stage of planning an endovascular intervention3,4 (Fig. 60-10). Traditionally, invasive angiography was used to determine the extent and severity of obstructive disease. Conventional angiography can use lower frame rates than needed for coronary angiography because most peripheral arteries are relatively static. Digital subtraction images remove bone and soft tissue from the image while leaving the contrast-enhanced image of the artery for more clarity, provided that the subtracted images remain still.
Noninvasive imaging is increasingly being used to plan vascular access and the tools probably required for the procedure. This includes magnetic resonance (MR) imaging using gadolinium or other contrast agents or time-of-flight techniques.31 Time-of-flight techniques rely on laminar blood flow and have the advantage of not requiring contrast material, which can rarely cause serious adverse effects (e.g., nephrogenic sclerosing fibrosis, see Chapter 17). But time-of-flight techniques may overestimate the severity of disease in regions of disturbed flow near obstructive or nonobstructive plaque. Computed tomography (CT) angiography using iodinated contrast material provides more rapid imaging, but heavy calcification can mask stenoses and make interpretation of lesion severity more difficult.32,33 Iodinated contrast agents can cause hypersensitivity and other allergic reactions or impair renal function.
MR imaging cannot be used in patients who have retained ferric metals (e.g., most pacemakers, shrapnel). Most stents are compatible with MR imaging but leave a flow void that does not allow interpretation of obstructive disease. High-resolution contrast-enhanced CT scans can discern the patency of stents and are a better advanced imaging modality for assessing stent patency. Both MR imaging and CT tend to overestimate the severity of stenosis when compared with conventional invasive angiography.
Duplex ultrasound is very useful for imaging arteries in the limbs and the cervical arteries and veins. This modality does require considerable time to map out large arterial systems, however, hence the more common use of MR and CT angiography as noninvasive techniques to plan endovascular interventions.
Vascular Access
The most common vascular access for the lower limb is from the contralateral common femoral artery. A catheter is directed from the access side over the bifurcation of the aorta and into the target iliac arteries via a support wire. A sheath is directed up and over the aortic bifurcation and pointed into the target iliac artery (Fig. 60-11). This approach is familiar to many operators and can be used to access the common femoral artery at its most superficial location. It also allows compression of the artery against the femoral head to aid in manual hemostasis after removal of the sheath.
The anterograde femoral approach involves skin access several centimeters cranial to the common femoral artery and angles toward the femoral head (see Fig. 60-11). This approach offers greater pushability for total occlusions and is closer to distal tibial lesions, but it is difficult in overweight patients, in whom the access needle must traverse a large distance through subcutaneous fat.
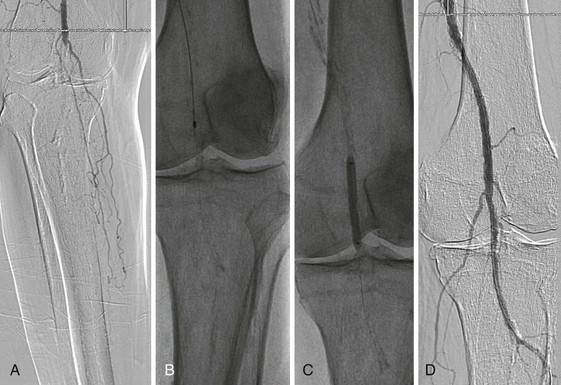
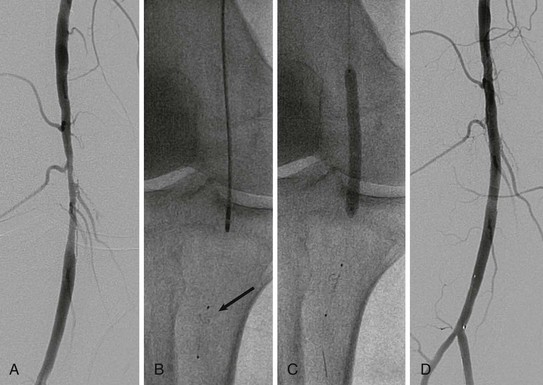
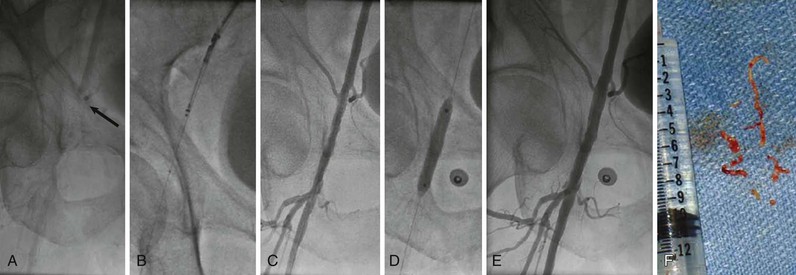
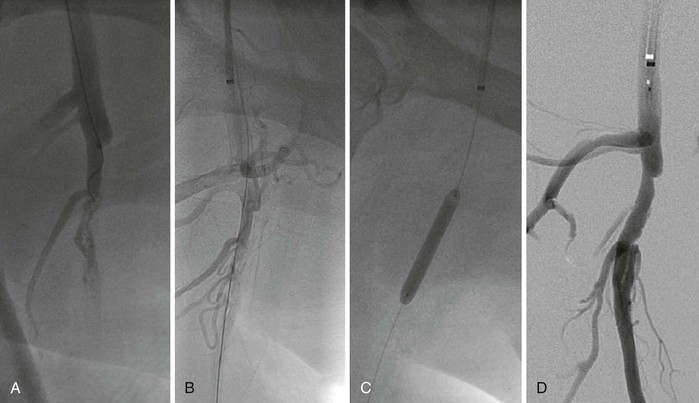
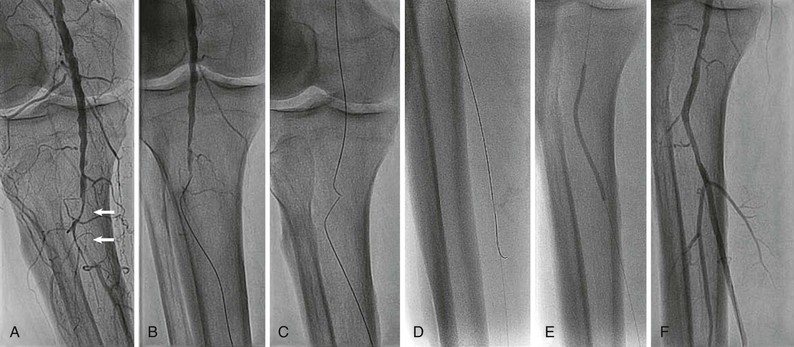
Rarely, retrograde access from the popliteal artery or from a tibial artery can assist in crossing a total occlusion that cannot be crossed from an anterograde approach34–36 (Figs. 60-12 and 60-13). The disadvantages of retrograde access are the potential to cause injury to the distal access site because of the smaller artery size (tibial arteries) or more difficult hemostasis from a deeper location (popliteal). Techniques that combine retrograde and anterograde approaches can assist in crossing difficult total occlusions. An unsuccessful procedure from a retrograde access site, however, could lead to a nonhealing ulcer and critical limb ischemia, and for this reason it is generally used as a “last resort.”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree