The presentation of acute pulmonary thromboembolism (PE) can be highly variable resulting in diagnostic challenges and management difficulties. Current guidelines suggest that therapy must be adjusted based on the severity of PE presentation. Systemic thrombolysis is the standard therapy for acute massive PE; however, systemic thrombolysis carries an estimated 20% risk of major hemorrhage, including a 3% to 5% risk of hemorrhagic stroke. There are data supporting the use of catheter-directed therapy (CDT) in massive and submassive PE, but past studies have limited its use to patients in whom systemic thrombolysis has either failed or was contraindicated. There is a paucity of data comparing the efficacy of CDT compared to systemic thrombolysis in different risk groups. This review will summarize the available data on the techniques and indications and outcomes of CDT for acute PE.
Acute pulmonary thromboembolism (PE) results in an estimated 150,000 deaths per year in the United States. On the basis of the data from major registries of patients with PE, it has been well established that mortality of PE depends on its clinical presentation. The 3-month mortality rate in patients who present with massive pulmonary embolism is approximately 50%, with an estimated overall mortality rate of around 15% in all patients who present with pulmonary embolism. The mainstay of therapy for PE is anticoagulation. In addition, present data suggest intravenous thrombolytic therapy for hemodynamically unstable patients or patients with evidence of significant right ventricular dysfunction or marked myocardial necrosis. Although, systemic thrombolysis is associated with reduced all-cause mortality rates compared to anticoagulation alone, it is associated with increased risk of major bleeding (9.2% vs 3.4%) and a 2% risk of intracranial bleeding. Therefore, only about 1/3 of candidates for thrombolysis receive systemic thrombolysis therapy in the United States mainly because of increased bleeding risk.
We sought to summarize contemporary evidence on emerging techniques, indications, and outcomes of catheter-directed therapy (CDT) for PE. Additionally, because ultrasound-accelerated thrombolysis (UAT) has been evaluated recently in prospective randomized trials, we present a proportion of meta-analysis designed to systematically evaluate prospective and retrospective cohort studies and assess the effects of UAT on all-cause mortality and major bleeding rates in patients with massive or submassive PE.
Management
Adjunctive systemic thrombolysis is the standard of therapy for high-risk patients with acute massive pulmonary embolism and hemodynamic instability. Systemic thrombolytic therapy has been proven by multiple studies to be superior to anticoagulation therapy only in dissolution of pulmonary emboli, faster restoration of pulmonary perfusion, and improved hemodynamics but limited evidence to support improvement in survival or prevention of recurrent PE. Systemic thrombolysis is associated with high risk of major bleeding complications (at least 10%) and intracranial bleeding (2% to 3%).
Currently, insufficient evidence exists to support systemic thrombolysis in hemodynamically stable patients, unless there is marked right ventricular dysfunction and/or major myocardial necrosis. The randomized double-blinded controlled trial, the Pulmonary EmbolIsm THrOmbolysis Study, investigated the benefit of fibrinolysis in 1,005 patients with intermediate-risk PE and reaffirmed prevention of hemodynamic decompensation at the expense of increased risk of major hemorrhage and stroke. Therefore, thrombolysis is reasonable to be used in the intermediate-risk group if there is low risk of bleeding.
Role of CDT
Several percutaneous interventions have been successfully introduced over the past years to remove proximal pulmonary emboli or to decrease thrombus burden aiming to decrease right ventricular afterload and rapidly reverse right ventricular failure and cardiogenic shock. CDT has the potential to offer similar clinical benefit as systemic thrombolysis with lower expected risk of major or intracranial bleeding because it uses mechanical methods for clot fragmentation or extraction and it enables a direct injection of lower-dose thrombolytic agents into the thrombus, which, theoretically, may decrease the risk of hemorrhagic complications compared with systemic thrombolysis. According to current scientific statement published by the American College for Chest Physicians and the American Heart Association in 2011, it is reasonable to use catheter-directed thrombolysis to target the risk group known to benefit from thrombolysis therapy when there is a contraindication for systemic thrombolysis or in patients who failed systemic thrombolysis and remain hemodynamically unstable. However, systemic thrombolysis has many absolute and relative contraindications. It is therefore not surprising that large portion of patients who present with massive or submassive PE have multiple medical co-morbidities that limit their eligibility for systemic thrombolysis. These patients may benefit from surgical embolectomy as the first line of therapy. Neily et al. in a cohort of 115 patients with massive or submassive PE (approximately 50% had contraindications to thrombolysis) showed low operative mortality rates with 20% 1-year mortality, suggesting that this method is safe and effective for selected patients with hemodynamically unstable PE and contraindications to thrombolysis. However, few tertiary care centers perform emergency surgical embolectomy for patients with massive PE, even when there are contraindications to thrombolysis. Therefore, CDT is preferred over surgical embolectomy in most centers with available clinical experience in performing CDT.
Several techniques of percutaneous catheter-directed intervention have evolved and become available to target high-risk patients presenting with PE: aspiration thrombectomy, thrombus fragmentation, rotational embolectomy, and rheolytic thrombectomy. In patients without absolute contraindication for thrombolysis, 2 approaches can be used at the same time: one of the conventional percutaneous catheter-based interventions listed previously in addition to catheter-directed thrombolysis. It is difficult to interpret the efficacy of the various interventional techniques because catheter intervention was combined with pharmacologic thrombolysis in most of the reported cases. A systematic review of 35 uncontrolled studies involving 594 patients presented the outcomes of catheter-directed therapy for pulmonary emboli. Overall, 33% of patients were treated with mechanical fragmentation and aspiration and did not receive thrombolysis. Efficacy (defined as restoration of hemodynamic stability, resolution of hypoxemia, and survival to discharge) was 86.5% and increased when thrombolytics were co-administered (91.2%). Minor complications were reported in 7.9% and major complications in 2.4%.
As presented in a recent analysis of the National Inpatient Sample database, utilization of CDT has increased since 2010. According to the same study, a propensity-matched analysis of a cohort of 352 inpatients with PE who underwent CDT compared to 352 inpatients who had systemic thrombolysis showed inhospital mortality rate of ∼10% which was significantly lower than the rates in systemic thrombolysis (∼20%), with very low rates of intracranial bleeding (0.28%) and no such cases after 2010, at the expense of higher rates of acute renal failure requiring hemodialysis and higher cost. The gradually decreasing major bleeding rates are probably reflective of improving experience and use of lower doses of thrombolytic agents with newer catheter-based devices. Despite increased utilization of CDT especially, there are limited data mainly from small series and cohort studies.
Role of CDT
Several percutaneous interventions have been successfully introduced over the past years to remove proximal pulmonary emboli or to decrease thrombus burden aiming to decrease right ventricular afterload and rapidly reverse right ventricular failure and cardiogenic shock. CDT has the potential to offer similar clinical benefit as systemic thrombolysis with lower expected risk of major or intracranial bleeding because it uses mechanical methods for clot fragmentation or extraction and it enables a direct injection of lower-dose thrombolytic agents into the thrombus, which, theoretically, may decrease the risk of hemorrhagic complications compared with systemic thrombolysis. According to current scientific statement published by the American College for Chest Physicians and the American Heart Association in 2011, it is reasonable to use catheter-directed thrombolysis to target the risk group known to benefit from thrombolysis therapy when there is a contraindication for systemic thrombolysis or in patients who failed systemic thrombolysis and remain hemodynamically unstable. However, systemic thrombolysis has many absolute and relative contraindications. It is therefore not surprising that large portion of patients who present with massive or submassive PE have multiple medical co-morbidities that limit their eligibility for systemic thrombolysis. These patients may benefit from surgical embolectomy as the first line of therapy. Neily et al. in a cohort of 115 patients with massive or submassive PE (approximately 50% had contraindications to thrombolysis) showed low operative mortality rates with 20% 1-year mortality, suggesting that this method is safe and effective for selected patients with hemodynamically unstable PE and contraindications to thrombolysis. However, few tertiary care centers perform emergency surgical embolectomy for patients with massive PE, even when there are contraindications to thrombolysis. Therefore, CDT is preferred over surgical embolectomy in most centers with available clinical experience in performing CDT.
Several techniques of percutaneous catheter-directed intervention have evolved and become available to target high-risk patients presenting with PE: aspiration thrombectomy, thrombus fragmentation, rotational embolectomy, and rheolytic thrombectomy. In patients without absolute contraindication for thrombolysis, 2 approaches can be used at the same time: one of the conventional percutaneous catheter-based interventions listed previously in addition to catheter-directed thrombolysis. It is difficult to interpret the efficacy of the various interventional techniques because catheter intervention was combined with pharmacologic thrombolysis in most of the reported cases. A systematic review of 35 uncontrolled studies involving 594 patients presented the outcomes of catheter-directed therapy for pulmonary emboli. Overall, 33% of patients were treated with mechanical fragmentation and aspiration and did not receive thrombolysis. Efficacy (defined as restoration of hemodynamic stability, resolution of hypoxemia, and survival to discharge) was 86.5% and increased when thrombolytics were co-administered (91.2%). Minor complications were reported in 7.9% and major complications in 2.4%.
As presented in a recent analysis of the National Inpatient Sample database, utilization of CDT has increased since 2010. According to the same study, a propensity-matched analysis of a cohort of 352 inpatients with PE who underwent CDT compared to 352 inpatients who had systemic thrombolysis showed inhospital mortality rate of ∼10% which was significantly lower than the rates in systemic thrombolysis (∼20%), with very low rates of intracranial bleeding (0.28%) and no such cases after 2010, at the expense of higher rates of acute renal failure requiring hemodialysis and higher cost. The gradually decreasing major bleeding rates are probably reflective of improving experience and use of lower doses of thrombolytic agents with newer catheter-based devices. Despite increased utilization of CDT especially, there are limited data mainly from small series and cohort studies.
Methods of Catheter-Directed Thrombolysis
Aspiration thrombectomy
The 10Fr Greenfield suction embolectomy catheterTM (Medi-tech/Boston Scientific, Massachusetts) was the first and the oldest introduced catheter-directed intervention ( Figure 1 , Table 1 ). The device removes the centrally located embolus by applying negative pressure to the tip using manual sustained suction with a large syringe. It requires the retrieval of the device and the thrombus as a unit. Conventional vascular access sheaths are not suitable for aspiration of large thrombus because it usually gets trapped within the sheath, so it has to be retrieved through surgical venotomy. The major disadvantage is the large lumen catheter, which has to be inserted through a venotomy through the femoral vein without a guidewire, and the catheter is difficult to manipulate because of its size and stiffness. A technique to avoid venotomy is to use a dedicated aspiration sheath with a detachable hemostatic valve, permitting percutaneous removal of thrombus without the need for surgical cut down.
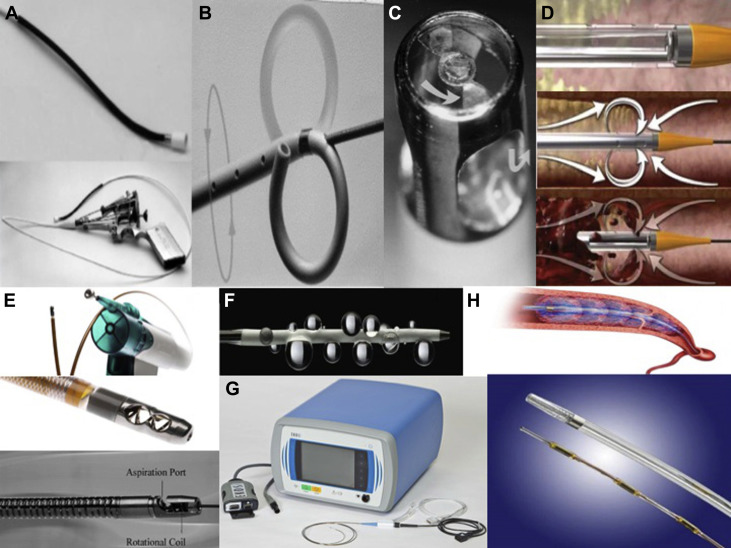
| Methods of CDT | Device name | Advantages | Disadvantages |
|---|---|---|---|
| Aspiration thrombectomy | Greenfield Suction embolectomy catheter™ (Medi-tec/Boston Scientific, USA) | Longest device experience | Large Lumen catheter required and catheter is difficult to manipulate due to size and stiffness |
| Thrombus Fragmentation | Pigtail rotational catheter™ (Cook-Europe, the Netherlands) | Increased risk of distal embolization | |
| Fogarty arterial Balloon embolectomy catheter™ (Edwards Lifescience Corp, Irvine, CA) | |||
| Amplatz-thrombectomy Device™ (ATD) | |||
| Rheolytic thrombectomy | AngioJet™ (MEDRAD, Warrendale, PA) | Not designed for the large sized main pulmonary arteries. Risk of arrhythmias | |
| Hydrolyser™ (Cordis, Miami, FL) | |||
| Oasis™ (Medi-tech/Boston Scientific, Natick, MA | |||
| Rotational Embolectomy | Rotarex™ | Does both fragmentation and aspiration | Prolonged aspiration may cause hemodynamic deterioration. |
| Rotational thrombectomy devices, Aspirex™ (Straub Medical, Wings, Switzerland) | |||
| Cleaner™ (Rex medical, Athens, TX) | |||
| Catheter directed thrombolysis | ClearWay™RX infusion catheter (Atrium Medical Corporation, Hudson, NH) | High success rate as a stand alone technique | Risk of major bleeding |
| Ultrasound accelerated thrombolysis | EkoSonic™ | Similar efficacy with smaller dose of thrombolytics | Lower treatment related complication and reduced thrombolytic infusion time. |
| EKOS® Catheter |
Thrombus fragmentation
Mechanical disruption of the thrombus into smaller fragments can be performed using manual rotation of a Pigtail rotational catheter (Cook-Europe, The Netherlands) or with peripheral Fogarty arterial Balloon embolectomy catheter TM (Edwards Lifescience Corp, Irvine, California). Balloon angioplasty using balloon sizes of 6 to 16 mm results in the compression of the embolus to the vessel wall but also in partial fragmentation of the thrombus with distal embolization. Amplatz catheter TM has been used, which uses an impeller to homogenize the thrombus. Most of the patients who have been treated with fragmentation techniques also received local thrombolysis. Therefore, it is unknown whether balloon angioplasty without concomitant thrombolysis is effective. The main disadvantage is the risk of distal embolization and further deterioration in hemodynamics when a large centrally located nonobstructive thrombus breaks and embolizes into distal branches.
Rheolytic thrombectomy
Rheolytic thrombectomy designed to remove intravascular thrombus, by applying Bernoulli’s principle relating to a low-pressure zone in the region of a high-velocity jet. Rheolytic thrombectomy catheters also called hydrodynamic thrombectomy devices include the AngioJetTM (MEDRAD, Warrendale, Pennsylvania), HydrolyserTM (Cordis, Miami, Florida), and OasisTM (Medi-tech/Boston Scientific, Natick, Massachusetts). These catheters have been used successfully for treatment of acute massive pulmonary embolism, using a high-velocity saline jet to fragment adjacent thrombus by creating a Venturi effect and removing the debris into an evacuation lumen. The main disadvantage of this technique is that it was not designed for the use in the large-sized main pulmonary arteries, thus may extract only a small amount of thrombus, rheolysis can result in profound and life-threatening arrhythmias, and small vessel perforation has been reported. On the basis of the available data, the tested hydrodynamic thrombectomy devices may cause perforations in vessels <6 mm in diameter.
Rotational embolectomy
These catheters designed to aspirate, macerate, and remove pulmonary artery thrombus using a high-speed rotational coil within the catheter body that creates negative pressure through an L-shaped aspiration port at the catheter tip. Commonly used catheters include the RotarexTM, AspirexTM rotational thrombectomy devices (Straub Medical, Wangs, Switzerland), and CleanerTM (Rex Medical, Athens, Texas). This technique is usually combined with catheter-directed thrombolysis. It combines the benefits of fine thrombus fragmentation with aspiration. Its downside is that prolonged aspiration may potentially cause hemodynamic deterioration in patients with PE-related shock because of blood loss.
Catheter-directed thrombolysis
Catheter-directed thrombolysis may be achieved with a variety of devices, including the ClearWayTM RX infusion catheter (Atrium Medical Corporation, Hudson, New Hampshire) with intrapulmonary injection of tPA or tenecteplase. This technique by itself may be successful; additional success is suggested when combined with other mechanical methods of thrombus fragmentation or aspiration. It requires positioning of an infusion catheter within the embolus, with injection of a bolus of thrombolytic drug, followed by a continuous infusion. Lower doses of the thrombolytic agent are used (average doses of tPA 10 to 20 mg vs systemic tPA infusion dose of 100 mg as a standard), Local delivery of the drug protects it from deactivation by circulating inhibitors and achieves higher drug concentration at the site of thrombus. The main complication is major bleeding; most bleeds occur at puncture site.
The recent Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT) prospective multicenter registry of 29 patients with massive and 71 patients with submassive PE administered standard CDT and thrombolytics in 64% of patients and UAT in the remaining 36% and reported 6% inhospital death, 0 major or intracranial bleeding events, and significant improvement of right-sided hemodynamics. In the PERFECT registry, the addition of UAT did not seem to offer any benefit over standard CDT.
Ultrasound-accelerated thrombolysis
UAT using the EkoSonicTM system is a novel treatment modality, more commonly used in some centers for treatment of acute massive PE. Ultrasound-enhanced thrombolysis uses high-frequency, low-power ultrasound. Ultrasound itself cannot dissolve thrombus but enhances fibrinolysis by causing reversible disaggregation of uncrosslinked fibrin fibers, thereby increasing thrombus permeability for the thrombolytic drug. Ultrasound pressure waves augment the penetration of thrombolytic drug. Compared with catheter-directed thrombolysis, this treatment technique provides similar treatment efficacy with reduced thrombolytic infusion time and treatment-related complications.
Our analysis identified 9 studies of ultrasound-assisted thrombolysis and 1 study which treated patients with CDT (64%) or UAT (36%) in patients with pulmonary embolism. The baseline characteristics of the included studies are summarized on Table 2 . All of them were reported from 2008 to 2015. These studies enrolled 469 patients who received UAT for treatment of PE with an average follow-up duration of 72 days. The mean age of patients was 59.5 years. With exception of 1 study that included only patients with massive PE, the remaining studies included ∼20% patients with massive and 80% patients with submassive PE. The primary outcome measure of our study was the development of all-cause mortality, severe and nonsevere bleeding as defined in the outcome section. The pooled clinical all-cause mortality was 3.6% (95% confidence interval 1.9% to 5.2%) without significant heterogeneity between studies ( Q = 9.4, p = 0.3 for heterogeneity; I2 0%; Figure 2 ). The pooled clinical major bleeding rate was 0.9% (95% confidence interval 0.1% to 1.8%) without significant heterogeneity between studies ( Q = 11.5, p = 0.12 for heterogeneity; I2 0%; Figure 3 ). The pooled mortality rates and the death rates in the most recent multicenter Ultrasound-Accelerated Thrombolysis of Pulmonary Embolism (ULTIMA) and EkoSonic Endovascular System and Activase for Treatment of Acute Pulmonary Embolism (SEATTLE II) studies (0% and 2.7%, respectively) are low and comparable with those observed in the previous large prospective controlled studies and lower than those reported in the National Inpatient Sample analysis of CDT. However, the main finding of our analysis is that the major bleeding rates were <1% which is lower that the rates reported with systemic thrombolysis which is ∼10%. Additionally, UAT resulted to hemodynamic improvement with lower postprocedural right ventricle/left ventricle ratio of ∼1 compared to preprocedural values.



