INDICATIONS
Bronchopleural fistula (BPF) is defined as an abnormal communication between a bronchus and the pleural cavity. Persistent air leaks originating from the lung parenchyma are not BPF and are properly designated alveolar-pleural fistula. BPF remains one of the most dreaded complications following pulmonary resection. BPF is uncommon occurring in 1% to 16% of patients undergoing pulmonary resection. Its incidence is greater following pneumonectomy than after lobectomy or segmental resection. Development of BPF has been significantly associated with the presence of benign disease, decreased preoperative FEV1, right pneumonectomy, and prolonged chest tube duration. Management of BPF continues to be a significant challenge particularly following pneumonectomy, and its presence has been associated with high morbidity and mortality. Management of postpneumonectomy BPF will be the focus of this chapter.
Fundamental to the prevention of BPF is careful attention to surgical details. Devascularization of the bronchus can occur following overly aggressive dissection. During pulmonary resection, leaving an excessively long bronchial stump predisposes to pooling of contaminated secretions and eventual stump dehiscence. There is increased risk of BPF when pulmonary resection is undertaken following radiation therapy or when pre-existing empyema is present. When such adverse conditions are present, the author and his colleagues prefer to reinforce the bronchial stump with viable tissue, most commonly an extrathoracic skeletal muscle such as serratus anterior.
Because the onset of infection is often insidious and occurs days to years following pulmonary resection, BPF diagnosis can be difficult. Patient presentation ranges from the nondescript findings of feeling chronically ill and having failure to thrive to the more obvious fever, chills, cough, purulent sputum, and chest discomfort. For pneumonectomy patients, a drop in the air–fluid level within the ipsilateral hemithorax especially when associated with expectoration of serosanguinous fluid is pathognomonic of BPF. Computed tomography (CT) can be helpful in evaluating the size and location of loculated air–fluid collections. Bronchoscopy should be performed inspecting the bronchial stump carefully for small BPF. The bronchial stump should be thoroughly irrigated to remove fibrin and mucus and gently probed though care taken to avoid inadvertent perforation.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Initial management of a patient with postpneumonectomy BPF depends on the clinical presentation. If the patient is acutely ill with respiratory distress and contralateral pneumonia, initial management starts with placing the patient in the lateral decubitus position, pneumonectomy side down until drainage of the pneumonectomy space is obtained. Chest tube placement in the pneumonectomy space should quickly be performed resulting in drainage of the cavity, stopping contralateral spillage, and beginning control of the infection. Appropriate resuscitation is undertaken and respiratory support provided as needed. Broad spectrum antibiotics begun and further adjusted when antimicrobial sensitivities become available. If a large BPF is present, mechanical ventilation may be required. A double-lumen endotracheal tube or long single-lumen tube carefully placed in the remaining lung’s mainstem bronchus is required to bypass the fistula, ventilate and decrease contamination of the remaining lung. Rarely is emergent surgery required. The author’s preferred treatment of postpneumonectomy BPF is a modification of the original Clagett procedure. The modification includes transposition of well-vascularized muscle to cover the bronchial stump to prevent recurrent fistula formation. Knowing which chest wall muscles were divided or otherwise utilized at the time of initial pulmonary resection is important. Usually the serratus anterior muscle is intact despite a previous thoracotomy. Other possible muscles for transposition include the pectoralis major and cephalic portion of the previously transected latissimus dorsi. Other options include the rectus abdominis and omentum. With the modified Clagett procedure, no attempt is made to completely fill the pleural cavity with either muscle or other autogenous tissue. Instead, the chest open chest cavity is filled with antibiotic solution at the time of skin closure.
Nutritional status is important in this group of patients who are often debilitated and malnourished from chronic infection. If necessary, nutritional supplementation including liberal use of enteral feedings should be considered in all of these patients.
 SURGERY
SURGERY
There are three basic principles to treatment: (1) Adequate pleural drainage, (2) closure of the fistula, and (3) obliteration of the residual pleural space. The author and his colleagues’ management of postpneumonectomy BPF has evolved over time. Our most common approach is two staged with each stage often containing multiple individual operative procedures. The first stage, again most commonly accomplished in a series of operations, consists of open drainage, closure of the BPF, muscle transposition, and debridement. Only after successful completion of the first stage is the second stage undertaken, which specifically includes obliteration of the pleural space with antibiotic solution and definitive closure of the chest wall.
All procedures begin with a team briefing. Surgeons, anesthesiologists, and operating room personnel are in attendance. Details of the airway management individualized for each specific patient are a major focus of the briefing. Patient identification, site marking, procedure verification, and final anesthetic evaluation are completed. Appropriate intravenous access is established. Epidural catheters are not utilized because of the risk of contamination. Instead, postoperative pain management most commonly is via a patient-controlled analgesia (PCA) pump. Following intubation, a urinary catheter and lower extremity sequential compression devices are placed. Subcutaneous injection of 5,000 units of unfractionated heparin is administered to reduce the risk of deep venous thrombosis (DVT) and pulmonary embolism.
Positioning
As long as the BPF is open, all procedures are done with isolation of the remaining lung via a long single-lumen endotracheal tube or double-lumen tube. All procedures are done in an open fashion, in a lateral decubitus position with the pneumonectomy side up. The exception being if the rectus abdominis muscle or omentum is harvested whereby the patient is in a supine position for that particular portion of the procedure.
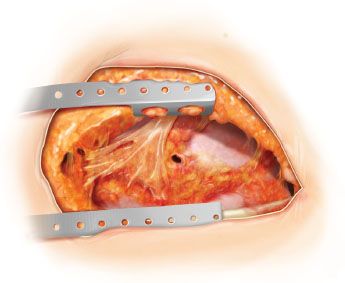
Figure 49.1 Open pleural drainage. The entire previous thoracotomy incision is reopened. A small bronchopleural fistula is seen.
First Stage
Open Pleural Drainage, Closure of the Fistula, Muscle Transposition, Chest Cavity Debridement
Technique
 Entire ipsilateral posterolateral thoracotomy is reopened (Fig. 49.1).
Entire ipsilateral posterolateral thoracotomy is reopened (Fig. 49.1).
 Chest cavity is debrided, appropriate specimens are sent for microbiologic and histopathologic testing.
Chest cavity is debrided, appropriate specimens are sent for microbiologic and histopathologic testing.
 BPF is sought by filling pleural cavity with saline and observing for escape of air bubbles from the mediastinum with positive pressure ventilation. This testing requires appropriate adjustment of the double-lumen tube or repositioning of the long single-lumen endotracheal tube.
BPF is sought by filling pleural cavity with saline and observing for escape of air bubbles from the mediastinum with positive pressure ventilation. This testing requires appropriate adjustment of the double-lumen tube or repositioning of the long single-lumen endotracheal tube.
 Small BPFs do not need to be closed at the time of initial debridement but instead can be repaired later when the pleural cavity is cleaner (usually at the second or third operation).
Small BPFs do not need to be closed at the time of initial debridement but instead can be repaired later when the pleural cavity is cleaner (usually at the second or third operation).
 If a large significant fistula is present, direct closure at the time of initial thoracotomy and debridement is preferred to prevent loss of ventilation and minimize ongoing contralateral contamination (Fig. 49.2A).
If a large significant fistula is present, direct closure at the time of initial thoracotomy and debridement is preferred to prevent loss of ventilation and minimize ongoing contralateral contamination (Fig. 49.2A).
 A long bronchial stump if present is mobilized into the mediastinum, redivided close to the carina with either staples (TA or TX 30 4.8-mm stapler) or sutured with interrupted 3-0 or 4-0 nonabsorbable monofilament suture (Fig. 49.2B).
A long bronchial stump if present is mobilized into the mediastinum, redivided close to the carina with either staples (TA or TX 30 4.8-mm stapler) or sutured with interrupted 3-0 or 4-0 nonabsorbable monofilament suture (Fig. 49.2B).
 This dissection can be hazardous because of proximity of the bronchus to the pulmonary artery and esophagus.
This dissection can be hazardous because of proximity of the bronchus to the pulmonary artery and esophagus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


