INDICATIONS
Pulmonary segmentectomy is most commonly used to treat patients with peripheral primary bronchogenic lung cancers less than 2 cm in size. Historically, segmentectomy was first described as a surgical treatment for bronchiectasis and tuberculosis. In 1939, Churchill and Belsey described anatomic segmentectomy for bronchiectasis of the lingua of the left upper lobe. These benign disease processes tended to occur bilaterally in the sublobar segments of the lung and were therefore well suited to a lung-sparing approach to resection. Over time, the procedure of segmentectomy became defined as the operative ligation of each individual bronchopulmonary segmental artery, vein, and bronchus, division of the lung parenchyma along the intersegmental plane, and in oncologic cases, the clearance of segmental, hilar, and mediastinal lymph nodes.
In the United states, by the end of the 1960s in men and the 1990s in women, lung cancer had become the most common form of cancer related death. Until the 1960s, pneumonectomy was regarded as the standard surgical treatment for lung cancer. However, the mortality rate associated with pneumonectomy for non-small cell lung cancer, first described by Graham in his groundbreaking series from 1933, approached 30% to 40%. In addition, the significant morbidity of pneumonectomy spurred interest in lesser more anatomic resections. Shimkin’s landmark comparison in 1962 of the Ochsner and Overholt clinics’ results comparing pneumonectomy and lobectomy for the treatment of lung cancer, clearly illustrated that patients with localized lung cancer had improved survival compared to those with more advanced disease, regardless of whether lobectomy or pneumonectomy was performed. Lobectomy with systematic lymph node dissection then became the standard operation for lung cancer. Controversy regarding the extent of resection and interest in lung-sparing procedures continued into the 1970s when Jensik et al. first established that anatomic segmentectomy provided an equivalent 5-year survival compared to lobectomy in stage I lung cancer patients. In response to this controversy, during the 1980s, the Lung Cancer Study Group (LCSG) performed the only randomized controlled trial to-date comparing survival outcomes after lobectomy or sublobar resection, which included segmentectomy and nonanatomic wedge resection in the treatment of early (cT1N0, stage IA) nonsmall cell lung cancer. This seminal study, published in 1995, concluded that lobectomy offered statistically significant superior control of local recurrence; yet no statistically significant survival benefit. However, the threefold increase in local recurrence rate found in the sublobar group and the trend toward decreased survival established lobectomy as the gold standard. Segmentectomy was considered a “compromise” operation for patients with limited cardiopulmonary reserve.
More recently, the controversy regarding sublobar resection and segmentectomy has again resurfaced as the diagnosis of small (less than 2 cm), peripheral, multiple, and subsolid nodules has increased dramatically with the advent and common use of CT scans of the chest. Furthermore, the diagnosis of smaller and less solid nodules has increased with the implementation of lung cancer screening programs. The characteristics of lung nodules diagnosed presently are significantly different than the lung cancers diagnosed in the LCOG study which relied on chest x-ray for diagnosis and allowed lesions up to 3 cm in size to be included in the study. Recent changes in the WHO classification of lung adenocarcinomas into adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and solid invasive carcinoma, reflect the profound heterogeneity in size, density, and invasive behavior seen in lung cancers diagnosed today using more modern approaches. In Japan, the early use of lung cancer screening programs and the adoption of sublobar resection for detected early stage lung cancers have provided significant data illustrating equivalent survival outcomes with segmentectomy and lobectomy. The Japanese experience illustrates that in appropriately selected patients, even those eligible for lobectomy, intentional sublobar resection, and in particular, segmentectomy, can provide equivalent long-term survival compared to lobectomy. Wedge resection has consistently been shown to offer inferior results in terms of local control and survival compared to both lobectomy and segmentectomy.
Currently, segmentectomy, either performed as an open or VATS procedure, is most indicated in patients with small, less than 2 cm (T1aN0M0 stage IA) peripheral lung cancers with limited pulmonary reserve who cannot tolerate formal lobectomy. In these patients, a segmentectomy will provide the most accurate oncologic staging and best overall outcomes compared to wedge resection, radiofrequency ablation, and radiation therapy. Recent studies have shown the feasibility, safety, and efficacy of segmentectomy as a primary treatment for early lung cancers regardless of cardiopulmonary risk. Furthermore, patients with synchronous primary lung cancers or metachronous cancer after previous lung resection should be considered for segmentectomy.
VATS segmentectomy is a technically challenging and demanding operation. The technical difficulty of performing a segmentectomy, either open or VATS, compared to a lobectomy, has likely contributed to the lack of widespread adoption of the procedure. In addition, the complexity of performing a VATS segmentectomy is considerable compared to open segmentectomy and VATS lobectomy. Some studies have shown higher complication rates; further limiting its use outside of expert centers with high-volume and high-expertise VATS programs. Over the past two decades, VATS Lobectomy has been proven to offer equivalent oncologic outcomes compared to open lobectomy. The long-term safety and equivalence in outcomes of a VATS approach for treating lung cancer has increased interest in VATS segmentectomy as a definitive treatment for early stage lung cancer. The benefit of a minimally invasive approach includes a shorter hospital length of stay, improved cosmesis, decreased postoperative pain, better postoperative pulmonary function and pulmonary toilet, and an increased tolerance of adjuvant chemotherapy, compared to open thoracotomy. In addition, VATS segmentectomy preserves healthy lung tissue and reduces the loss of pulmonary function. This is critical for patients who have undergone previous lung resection, have minimal pulmonary reserve, and otherwise would not be candidates for resection, or in patients who have or are at risk of developing multiple lung cancers.
 CONTRAINDICATIONS
CONTRAINDICATIONS
VATS segmentectomy should not be considered in patients with tumors larger than 2 cm (any T1b or greater T stage tumor) or in whom there is evidence of nodal disease, in effect, precluding any lung cancer staged greater than AJCC seventh edition T1aN0M0, stage IA. VATS segmentectomy should not be considered in patients with central tumors close to the origin of the lobar bronchi, or in tumors with endobronchial invasion, as an appropriate margin will require a more extensive resection that is accomplished more readily with an anatomic lobectomy. One can consider performing an extended VATS segmentectomy, that includes additional parenchyma from a neighboring segment without hesitation, or a formal segmentectomy of multiple segments. However, the amount of viable lung tissue preserved after removing multiple segments would be questionable and the risk of complications, such as torsion of the unresected segment, higher compared to a lobectomy. Hilar and mediastinal lymph nodes must be examined at the time of surgery and any lymph node metastasis is an indication to convert the operation to a formal lobectomy.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
All patients must have dedicated imaging of the chest with a CT scan, including the adrenal glands, using 3-mm intervals or less per image. Contrast enhanced scans are not necessary but can be helpful in providing specific information regarding the size and location of lymph nodes, the anatomic location of critical vessels, and the relationship of the tumor to vascular structures that is helpful for preoperative planning and segmental localization. In addition, reformatted images of axial, sagittal, and coronal views are imperative for determining the exact segmental location of the lesion. Margins of at least 2 cm must be planned for adequate oncologic outcomes and the precise location of the tumor is essential for guaranteeing an appropriate surgical approach. In addition, variations in the anatomy of the right upper lobe are common, and detailed preoperative knowledge of the arterial, venous, and bronchial anatomy is required for a safe and effective operation. A preoperative CT-guided biopsy is helpful for lesions more centrally located, are nonsolid, or are located deeper than 2 cm from the pleural edge because a wedge resection for diagnosis may alter the lung parenchyma to a degree that VATS segmentectomy will be difficult to complete. If the lesion is superficial, or located within a large segment with enough surrounding parenchyma, an intraoperative wedge resection for diagnosis is commonly performed to confirm the diagnosis of cancer followed by a completion segmentectomy done in the same sitting for definitive therapy. A diagnostic and therapeutic VATS segmentectomy is also acceptable, but VATS segmentectomy is considerably more technically challenging than wedge resection or lobectomy, and the risk of complications intraoperatively or postoperatively may not justify this as a common approach to the diagnosis and treatment of lung nodules.
A preoperative PET/CT scan must be done to clinically stage the patient as accurately as possible. PET uptake in hilar or mediastinal lymph nodes should be evaluated with Endobronchial ultrasound—transbronchial needle aspiration (EBUS-TBNA) or mediastinoscopy as up to 7% of lung cancers less than 1 cm in size will have lymph node metastasis. One must bear in mind that most tumors considered for segmentectomy are small and subsolid. These lesions may not show PET avidity and a negative PET must not be considered proof of lack of malignancy as the resolution of PET is diminished in small and less dense lesions.
Many patients considered for segmentectomy will have marginal cardiopulmonary function and a complete cardiopulmonary evaluation must be completed prior to surgery. Pulmonary function tests (PFTs) with forced expiratory volume in 1 second (FEV1) and DLCO (diffusion of carbon monoxide) are essential for documenting the patient’s respiratory reserve. A calculation of postoperative predicted FEV1 and DLCO can be made by assigning roughly 5% of perfusion to each segment. However, attention should be paid to the degree of pulmonary dysfunction because conversion to lobectomy may become necessary in some patients at the time of surgery. In general, most patients will be evaluated with a cardiac stress test to quantify their cardiac risk for the procedure. Additional investigation with split lung function and ventilation–perfusion testing, may help in the accurate calculation of expected loss of pulmonary function. Patients with borderline data points should undergo cardiopulmonary exercise testing (CPET) to determine the VO2 max. Patients with VO2 max values greater than 20 mL/kg/min are considered safe for pulmonary surgery.
Although not necessary in many cases, preoperative tumor localization and marking with methylene blue dye or metallic fiducial markers can be helpful in locating small, nonsolid, or deep nodules at the time of surgery. Navigational bronchoscopy technology that merges GPS capabilities with thin-cut CT imaging provides a useful method for marking small and subsolid tumors that may be difficult to palpate intraoperatively using a VATS technique. Fiducial markers and the injection of blue dye using navigational bronchoscopy can add tactile and visual aids for localizing and successfully removing these lesions. In addition, the three-dimensional software used for navigational bronchoscopy planning enables the most accurate determination of the segmental location. The ability to view the three-dimensional location of the nodule is especially helpful for lesions that exist close to the border of adjacent segments because it allows for the planning of surgical margins and an extended resection. CT-guided marking with fiducials and dye is also feasible but requires a separate outpatient procedure with added patient inconvenience.
 SURGERY
SURGERY
Positioning
Following the induction of general anesthesia, a single-lumen endotracheal tube is placed first. A diagnostic bronchoscopy is performed to confirm the bronchial anatomy and reveal any variations that will affect either the operative plan or control of the airway for single lung ventilation. Next, a double-lumen endotracheal tube is used to establish single lung ventilation. This allows for bronchoscopic confirmation of airway anatomy during the case with a pediatric bronchoscope. An arterial line is placed along with two large-bore peripheral IVs for access and beat-to-beat monitoring. EKG pads are placed strategically away from the sterile field. The patient is placed in the left lateral decubitus position with the right side up. The patient is placed in a flexed position to increase the aperture of the rib spaces. An axillary roll is placed and all pressure points adequately padded to avoid nerve injury. The right arm is placed on a padded arm board with the elbow flexed gently toward the head to open the space under the axilla.
Port Sites
A standard three port VATS technique is used for each right upper lobe segment. The camera port consists of a 10-mm incision in the seventh intercostal space, midaxillary line. A posterior 10-mm port is placed inferior and posterior to the scapular tip and is used for retraction of the lung and the stapling device for all of the hilar structures. A 3- to 4-cm utility incision is placed in the fourth intercostal space, starting from the anterior border of the latissimus dorsi muscle toward the ventral chest wall in the direction of the interspace (Fig. 22.1). Port placement can be varied as needed to accommodate each individual patient’s anatomy and surgeon’s comfort. The correct angle of approach and distance from the right upper lobe hilum for VATS instruments is critical for a safe, efficient, and reproducible operation and the exact location of port sites may change with differing patient anatomy and body habitus. In general, the camera port site should allow for a panoramic view of the entire chest but be located anteriorly enough that the entire anterior aspect of the hilum can be visualized during the dissection of each anatomic structure. At the same time, visualization of the posterior hilum is also necessary for posterior segmentectomy. Either a 10- mm or 5-mm 30-degree–angled scope is used for the case. The angled scope is essential for providing safe visualization of the hilar structures. The utility incision should be placed directly above the superior pulmonary vein and should allow for a slight, 10-degree tilt, of the instruments when approaching the hilum from the operating surgeon’s side, which is the patient’s anterior chest.
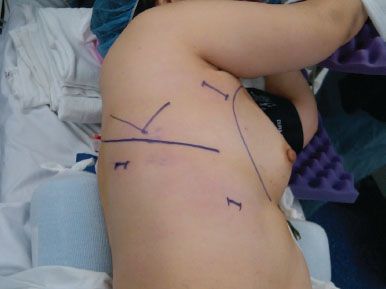
Figure 22.1 Patient positioning and port site placement (JPEG): The patient is placed in the left lateral decubitus position. The camera port is placed in the seventh intercostal space, midaxillary line. The posterior port site, used for lung retraction and stapling devices, is placed below and posterior to the tip of the scapula. The utility incision is 3 to 4 cm in length and starts just anterior to the border of the latissimus dorsi muscle near the axillary crease.
Technique
Anatomy
A thorough and complete knowledge of the segmental anatomy of the right upper lobe is essential for performing a safe and effective VATS segmentectomy. The right upper lobe is divided into three individual segments, an apical, a posterior, and an anterior segment. To perform each segmentectomy with a VATS approach, the surgeon must have a detailed understanding of each individual segmental bronchial, arterial, and venous anatomy and their common variations. Segmental anatomy is less commonly explained or detailed in most anatomy textbooks or surgical atlases and the reader’s knowledge of the three-dimensional positions of these structures may not be as substantial as lobar anatomy. From the lateral surgical view of the lung, the surgeon can visualize each of the three right upper lobe segments, the posterior, apical, and a portion of the anterior segment of the right upper lobe (Fig. 22.2A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


