Transposition of the Great Vessels
Transposition of the great arteries is a congenital malformation in which the heart has atrioventricular concordance and ventriculoarterial discordance. Therefore, the clinical findings are an anterior aorta that originates from the morphologic right ventricle and a pulmonary artery that originates from the morphologic left ventricle. Other congenital defects can also be associated with transposition of the great arteries.
Today, anatomic correction of transposition of the great arteries with or without ventricular septal defect is the procedure of choice. When the interventricular septum is intact, the arterial switch operation must be performed while the left ventricle is still prepared to handle systemic pressures. After 2 to 3 weeks of age, changes in the left ventricular wall thickness and geometry may preclude a successful arterial switch procedure. If the left ventricular pressure is less than 60% systemic, a two-staged approach involving initial pulmonary artery banding with or without a systemic to pulmonary artery shunt followed by an arterial switch procedure when the left ventricle becomes prepared is required. Alternatively, a so-called atrial switch procedure (Mustard or Senning operation) may be undertaken.
The Senning and Mustard procedures were designed to achieve a rerouting of the venous returns in the two atria; this entails channeling the systemic venous return from the caval veins into the left atrium, across the mitral valve into the left ventricle, and through the pulmonary artery to the lungs. Similarly, pulmonary venous return from the pulmonary veins is directed into the right atrium across the tricuspid valve into the right ventricle, which functions as the systemic ventricle, pumping blood into the aorta. Except for the torn fossa ovalis, which is found if a palliative balloon septostomy has been performed, the surgical anatomy of both the right and left atria is essentially normal. The long-term follow-up of patients who have undergone a Senning and Mustard procedure has shown a high incidence of atrial arrhythmias and a significant rate of late right ventricular dysfunction. However, physiologic repair with one of these two procedures may be indicated in patients with transposition of the great vessels and associated pulmonary valve stenosis, nonresectable left ventricular outflow tract obstruction, or some abnormalities of the coronary arteries that may prohibitively increase the risk of anatomic repair. An atrial switch procedure may be part of the surgical approach in patients with some complex congenital heart lesions, and therefore every surgeon dealing with patients with congenital heart disease should have the Senning and Mustard procedures as part of his or her surgical armamentarium.
Surgical Anatomy
In hearts with transposition of the great arteries, the right ventricular wall thickness is greater than normal at birth and increases progressively thereafter. If the ventricular septum is intact and no pulmonary stenosis exists, the left ventricular wall thickness does not increase after birth, and within 2 to 3 months, the left ventricle is relatively thin walled.
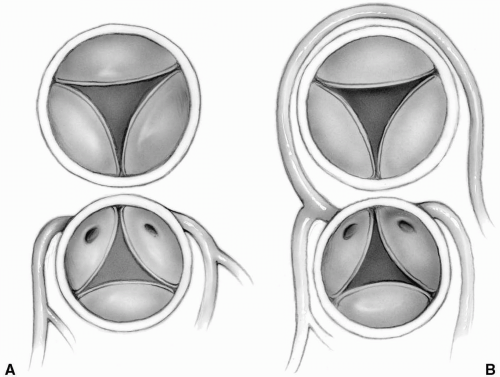
The aorta is most commonly directly anterior to the pulmonary artery, although occasionally the great vessels are side by side with the aorta to the right. The coronary arteries usually arise from the aortic sinuses facing the pulmonary artery. Therefore, the nonfacing sinus is most often anterior. According to the Leiden convention, sinus 1 is on the right-hand side and sinus 2 is the next sinus counterclockwise to sinus 1, as viewed from the nonfacing noncoronary sinus. Approximately 70% of patients have the left anterior descending and circumflex coronary arteries arising as a single trunk from sinus 1 and a right coronary artery from sinus 2 (Fig. 25-1A). The left anterior descending arises from sinus 1, and the right coronary artery and circumflex originate together from sinus 2 in approximately 15% of cases (Fig. 25-1B). Rarely, all three main coronary arteries arise from a single sinus, most commonly sinus 2. In some of these cases, the left anterior descending or left main coronary artery may be intramural.
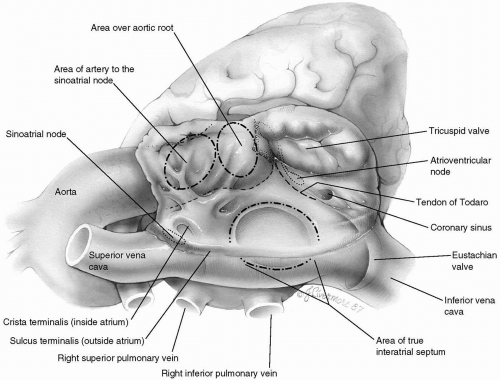
Surgical Anatomy of the Right Atrium
Although the right atrium is morphologically molded into a single chamber, it is formed by two components: the sinus venarum and the right atrial appendage (sometimes referred to as the body of the atrium). Systemic venous return
flows in from opposite directions through the superior and inferior venae cavae into the sinus venarum. This smooth-walled area is the most posterior portion of the right atrium and stretches between the orifices of the caval veins. From the viewpoint of the surgeon looking down into the right atrium, the sinus venarum is more or less horizontal, with the superior vena cava entering from the left and the inferior vena cava entering (bounded by the eustachian valve) from the right (Fig. 25-2).
flows in from opposite directions through the superior and inferior venae cavae into the sinus venarum. This smooth-walled area is the most posterior portion of the right atrium and stretches between the orifices of the caval veins. From the viewpoint of the surgeon looking down into the right atrium, the sinus venarum is more or less horizontal, with the superior vena cava entering from the left and the inferior vena cava entering (bounded by the eustachian valve) from the right (Fig. 25-2).
Just below and medial to the orifice of the superior vena cava arises the crista terminalis, a muscle bundle that springs into prominence as it circles the orifice of the superior vena cava to the right lateral wall of the atrium and continues inferiorly toward the inferior vena cava, thereby forming the boundary between the sinus venarum and the atrial appendage. This muscle bundle is evidenced on the outside of the atrium by a groove, the sulcus terminalis. Lying subepicardially in the sulcus terminalis, just below the entrance of the superior vena cava, is the sinoatrial node, which may be vulnerable to injury from the various surgical incisions and cannulations that are commonly performed on the right atrium. The remainder of the right atrium is made up of atrial appendage, which begins at the crista terminalis and extends anteriorly (upward from the surgeon’s perspective) to surround the tricuspid valve and form an expanded chamber.
In contrast to the smooth-walled sinus venarum, the lateral wall of the atrial appendage is ridged by multiple narrow bands of muscle, the musculi pectinati. These bands arise from the crista terminalis and pass upward to the most anterior part of the atrium. Functionally, they supply the right atrium with enough pumping capacity to propel the venous inflow through the tricuspid valve into the right ventricle.
Just above the sinus venarum in the center of the medial wall is the fossa ovalis, an elliptic or horseshoe-shaped depression. The true interatrial septum consists of the fossa ovalis with variable contributions from the superior, anterior, and inferior limbic muscle bundles that surround it. The aortic root is hidden behind the anteromedial atrial wall between the fossa ovalis and the termination of the heavily trabeculated right atrial appendage. Segments of the noncoronary and right sinus of Valsalva are in close apposition to the atrial wall in this area. Their locations may be manifested by the aortic mound, a bulge above and slightly to the left of the fossa ovalis. The presence of the aortic valve here can be more clearly visualized if one takes into consideration its continuity, through the central fibrous body, with the adjacent tricuspid valve annulus.
Also invisible to the surgeon is the artery to the sinoatrial node, which runs through this same area. Although its origin and exact location are unpredictable, the artery to the sinoatrial node takes a variable course toward the superior cavoatrial angle and the sinus node.
The tricuspid valve is located anteroinferiorly in the right atrium, where it opens widely into the right ventricle. The annulus of the tricuspid valve crosses over the membranous septum, dividing it into atrioventricular and interventricular segments. The membranous, or fibrous, septum is a continuation of the central fibrous body, through which the tricuspid, mitral, and aortic valves are connected.
Immediately below the upper or atrioventricular section of the membranous septum lies the hidden atrioventricular node. It is situated at the apex of the triangle of Koch, the boundaries of which are the annulus of the septal leaflet of the tricuspid valve, the tendon of Todaro (running intramyocardially from the central fibrous body to the
eustachian valve of the inferior vena cava), and its base, the coronary sinus. Anderson describes the tendon of Todaro as a fibrous extension of the commissure between the eustachian valve (of the inferior vena cava) and the thebesian valve (of the coronary sinus). Conduction tissue passes from the atrioventricular node as the bundle of His below the membranous septum and down into the muscular interventricular septum. The coronary sinus, draining the cardiac veins, is situated alongside the tendon of Todaro, between it and the tricuspid valve.
eustachian valve of the inferior vena cava), and its base, the coronary sinus. Anderson describes the tendon of Todaro as a fibrous extension of the commissure between the eustachian valve (of the inferior vena cava) and the thebesian valve (of the coronary sinus). Conduction tissue passes from the atrioventricular node as the bundle of His below the membranous septum and down into the muscular interventricular septum. The coronary sinus, draining the cardiac veins, is situated alongside the tendon of Todaro, between it and the tricuspid valve.
The Arterial Switch Operation
Incision
A median sternotomy is performed, and the thymus is removed.
Preparation
A rectangular piece of pericardium is harvested and treated with glutaraldehyde. The relationship of the great vessels and coronary anatomy can be confirmed at this point.
Cannulation
The ascending aorta is cannulated as far distally as possible. Direct bicaval cannulation is carried out. With initiation of cardiopulmonary bypass, the ductus arterious is occluded at its aortic end with a heavy tie or metal clip. The ductus arteriosus is later divided, oversewing the pulmonary artery side with 6-0 or 7-0 Prolene suture. A vent is placed through the right superior pulmonary vein. During cooling, the ascending aorta is dissected free from the main pulmonary artery, and the right and left pulmonary arteries are mobilized out to the first branches in the hilum of each lung. Most or all of the dissection is accomplished with an electrocautery on low current.
As soon as cardiopulmonary bypass is instituted, the ductus arterious must be occluded to prevent runoff of aortic cannula flow into the lungs.
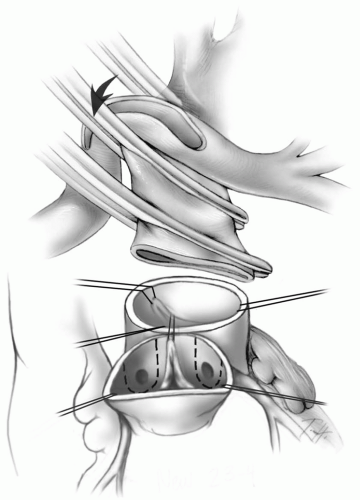
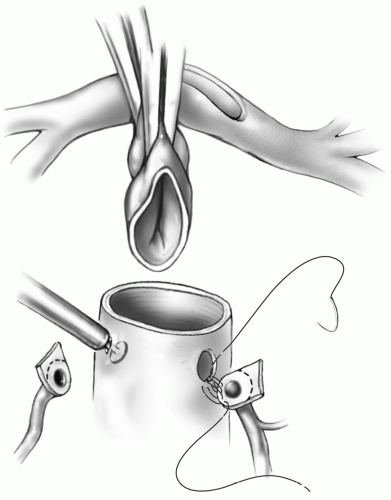
Transection of the Great Arteries
The aortic cross-clamp is applied just proximal to the aortic cannula. A dose of cold blood cardioplegic solution is administered through a butterfly needle into the ascending aorta approximately 1 cm above the valve. The aorta is then transected at this level, and traction sutures are placed just above the three commissures of the aortic valve and tagged (Fig. 25-3). The pulmonary artery is transected at the level of the takeoff of the right pulmonary artery, and
traction sutures are placed at the commissures and tagged. The pulmonary valve is inspected to rule out significant abnormalities because this will be the new aortic valve.
traction sutures are placed at the commissures and tagged. The pulmonary valve is inspected to rule out significant abnormalities because this will be the new aortic valve.
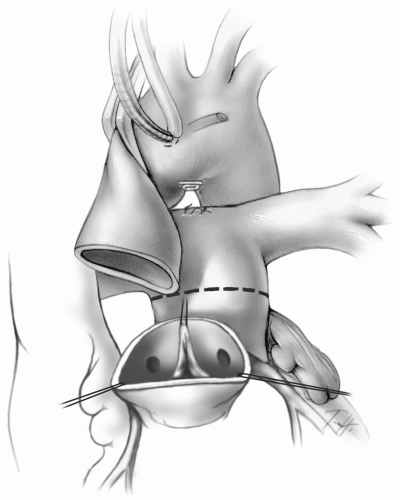 FIG 25-3. The ascending aorta has been transected. Note the divided ductus arteriosus and line of transection on the main pulmonary artery. |
The status of the pulmonary valve is usually defined by the preoperative transthoracic echocardiogram and intraoperative transesophageal echocardiogram.
A sufficiently competent and nonstenotic valve must be confirmed before excising the coronary arteries.
The pulmonary artery confluence is brought anterior to the distal ascending aorta (Fig. 25-4). The most proximal portion of the transected distal aorta is then grasped with a forceps or straight vascular clamp. The initial cross-clamp is then reapplied proximal to the pulmonary artery confluence as high as possible on the ascending aorta (Fig. 25-4B). This technique, referred to as the Lecompte maneuver after the surgeon who originally described it, avoids the need for an interposition conduit to connect the new pulmonary artery base to the pulmonary artery confluence.
When repositioning the aortic cross-clamp, care must be taken not to twist the aorta and create torsion at the aortic suture line.
The coronary ostia and at least 2 to 3 mm of surrounding aortic wall are excised as tongues of tissue (Fig. 25-4). The proximal coronary arteries are mobilized from the epicardium for several millimeters using an electrocautery on low current.
Adequate dissection of the coronary arteries must be carried out to allow successful translocation of each coronary ostium to the corresponding sinus of the pulmonary artery. Insufficient mobilization may lead to tension on the coronary anastomosis or kinking of the coronary artery.
Conal branches may rarely need to be ligated and divided to allow adequate mobilization of the right coronary artery.
When one or both coronary ostia arise immediately adjacent to the commissure, the adjacent commissure must be excised along with the coronary ostia. This may lead to mild neopulmonary valve insufficiency.
A generous cuff of aortic wall must be included in the tongue of tissue containing the coronary ostium to avoid injury to the intramural portion of the coronary artery.
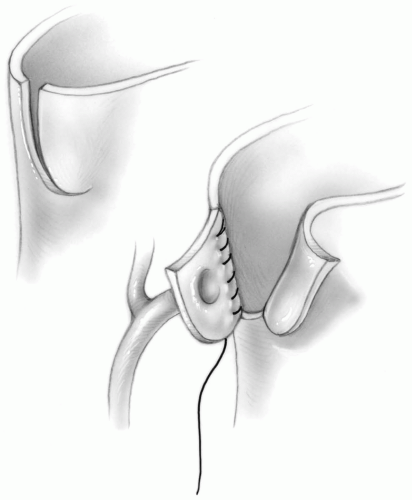
Coronary Artery Reimplantation
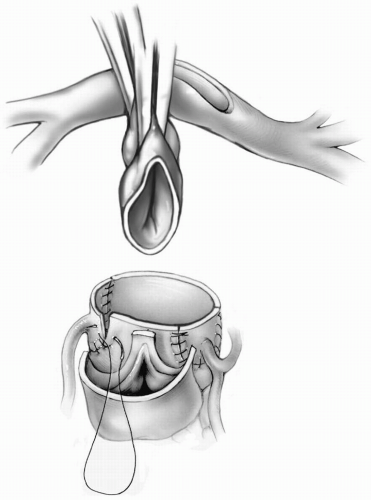
The reimplantation sites for the coronary ostia are determined by holding the mobilized coronary arteries up against the anterior facing sinuses of the pulmonary root, ensuring that no distortion of the proximal course of the coronary arteries is created. The coronary arteries can be reimplanted into the pulmonary root as tongues of tissue by making a U-shaped incision in the appropriate location (Fig. 25-5). Alternatively, the coronary flaps are reattached as buttons of tissue, trimming the distal end of the flap before completing the suture line. In this case, a small slit is made at the appropriate location for coronary reimplantation in the pulmonary root. A small aortic punch is introduced into this hole and used to create an appropriately sized opening. The coronary artery is sutured to the opening in the pulmonary root using 7-0 or 8-0 Prolene suture (Fig. 25-6). After each coronary anastomosis, cold blood cardioplegic solution is infused directly into each coronary ostium with a 2-mm olive-tipped cannula, allowing assessment of any kinking or distortion of the coronary artery. If any problems are detected, they should be rectified now by either freeing up any restrictive adventitial or epicardial bands or redoing the anastomosis.
Some surgeons prefer to excise the coronary ostia as buttons, instead of tongues of tissue from the aortic root. When this technique is used, extreme care must be taken to prevent rotation and distortion of the coronary artery button during reimplantation.
If the circumflex coronary artery arises from the right coronary artery, a trapdoor may be created in the neoaorta to prevent kinking of the takeoff of the circumflex branch (Fig. 25-7). Alternatively, the right coronary artery ostium may be implanted higher on the neoaorta. When the circumflex arises from the right coronary artery, the pulmonary artery should be transected as far distally as possible to allow a high reimplantation of the right coronary button. Occasionally, the anastomosis must be performed on the ascending aorta distal to the suture line joining the neoaortic root to the distal aorta (Fig. 25-8).
A shallow, U-shaped incision is made in the proximal neoaorta adjacent to the location of the previously prepared aortic tongue of tissue containing the involved coronary ostium or ostia. The upper edge of the aortic tongue is sutured to the lower portion of this U-shaped opening in the neoaortic root with a 7-0 Prolene suture (Fig. 25-9A). The suture line is secured at both ends. The neoaortic root is anastomosed to the ascending aorta posteriorly, securing the suture line on both sides where it meets the coronary artery anastomosis. A piece of autologous pericardium is cut in the appropriate shape, and sewn into place to create a convex roof over the remaining opening (Fig. 25-9B). This technique allows the coronary artery to maintain its original orientation, and minimizes the risk of twisting or tension on the proximal course of the coronary artery.
Care must be taken when making the opening in the neoaortic root first with the knife blade and then with the punch to protect the valve leaflets from injury. The assistant may gently retract the leaflet with the back of a fine forceps.
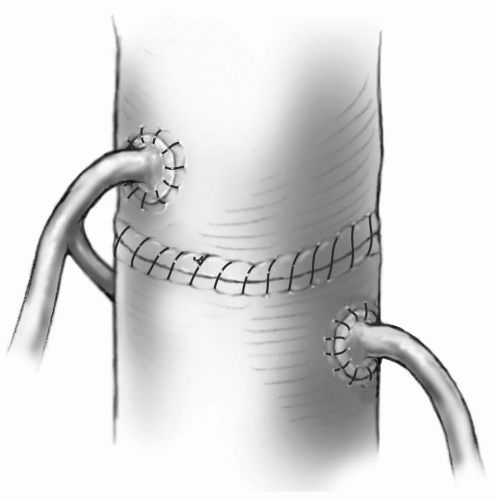
Reconstructing the Aorta
The distal ascending aorta is anastomosed to the neoaortic root with running 6-0 or 7-0 Prolene suture (Fig. 25-10).
If a discrepancy between the diameter of the distal ascending aorta and neoaortic root exists, the excess tissue can usually be gathered in the posterior suture line. Gathering tissue anteriorly can distort the coronary artery anastomoses. This is especially true if the coronary arteries have been reimplanted as flaps.
Care must be taken to ensure hemostasis of the aortic suture line, especially posteriorly, because this is relatively inaccessible after the repair is completed.
When the aorta and pulmonary artery are side by side, the Lecompte maneuver may not be performed.
The distal ascending aorta is mobilized laterally and anastomosed to the neoaortic base.
Intracardiac Repair
The atrial septal defect or balloon atrial septostomy and a ventricular septal defect, if present, are closed through a right atriotomy incision (see Chapters 19 and 21). Alternatively, a ventricular septal defect can be closed through one of the semilunar valves. If this is done through the posterior (pulmonary) valve, great care must be taken to avoid the conduction system. After closing the right atrium, the aortic cross-clamp may be removed or left in
place until the pulmonary artery reconstruction is finished. If it is left in place, another dose of cardioplegia is given through a butterfly needle in the neoaortic root, allowing the surgeon to check the lie and filling of the coronary arteries as well as the suture lines for bleeding.
place until the pulmonary artery reconstruction is finished. If it is left in place, another dose of cardioplegia is given through a butterfly needle in the neoaortic root, allowing the surgeon to check the lie and filling of the coronary arteries as well as the suture lines for bleeding.
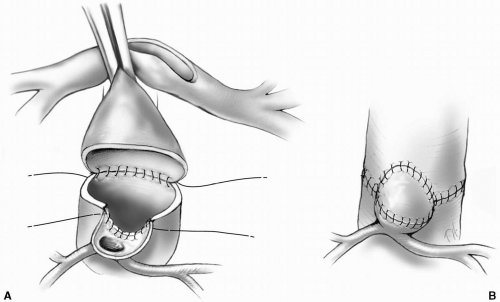 FIG 25-9. A: Anastomosing a single intramural coronary artery to the neoaorta maintaining the native orientation of the coronary ostium. B:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|