17 Transcatheter Valve Therapies
Recent advances in percutaneous-based catheter technologies have allowed for development of novel therapies for valvular heart disease, including transcatheter valve implantation and transcatheter mitral valve repair (Table 17-1). Although these devices are still investigational in the United States, many have incorporated transcatheter valve procedures into their daily practice. This chapter briefly reviews two transcatheter valve procedures: transcatheter aortic valve replacement and percutaneous edge-to-edge mitral valve repair.
Table 17-1 Transcatheter Valve Therapies
| Transcatheter Valve Implantation |
| Percutaneous Mitral Valve Repair |
Transcatheter Aortic Valve Replacement (TAVR)
Indications
Aortic valve replacement (AVR) is indicated in all symptomatic patients with severe AS (2006 ACC/AHA Valvular Heart Disease Guidelines, Class I recommendation, Level of Evidence: B). Despite this recommendation, a significant number of elderly patients (up to one third) do not undergo AVR because of comorbidities, including advanced age and the associated increased morbidity and mortality with surgery. Additionally, many elderly patients that are suitable surgical candidates will refuse surgery because they perceive themselves as “too old” to survive open AVR. Transcatheter aortic valve replacement (TAVR) is a promising alternative to surgical AVR in those patients deemed high risk for surgery (Table 17-2).
Table 17-2 Indications for TAVR
STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement.
Transcatheter Valves
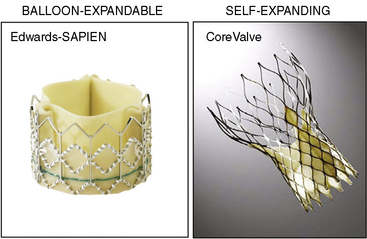
There are two catheter-implantable valves commercially available overseas. They are the balloon-expandable Edwards SAPIEN valve and the self-expanding Medtronic CoreValve (Fig. 17-1). The Edwards SAPIEN valve was studied in the PARTNER trial, which completed randomized enrollment in fall 2009. The Medtronic CoreValve randomized trial started enrollment in December 2010. The initial international experience and the results of PARTNER cohort A and B suggest that outcomes compare favorably with conventional valve surgery in selected patients. In November 2011, the Edwards SAPIEN valve was FDA approved and became commercially available in the United States for PARTNER cohort B (inoperable) patients.