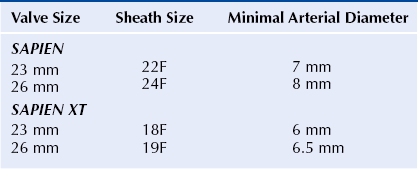
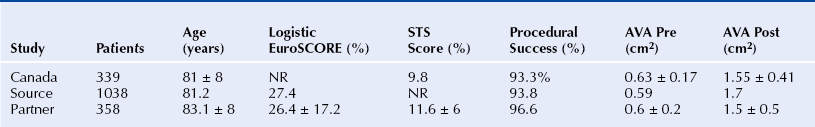
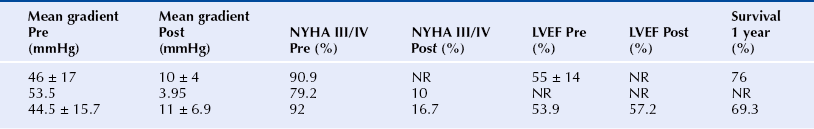
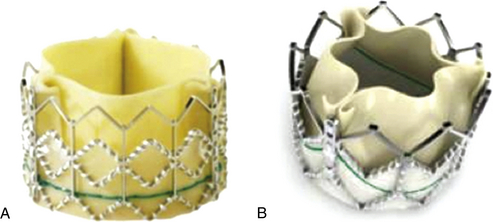
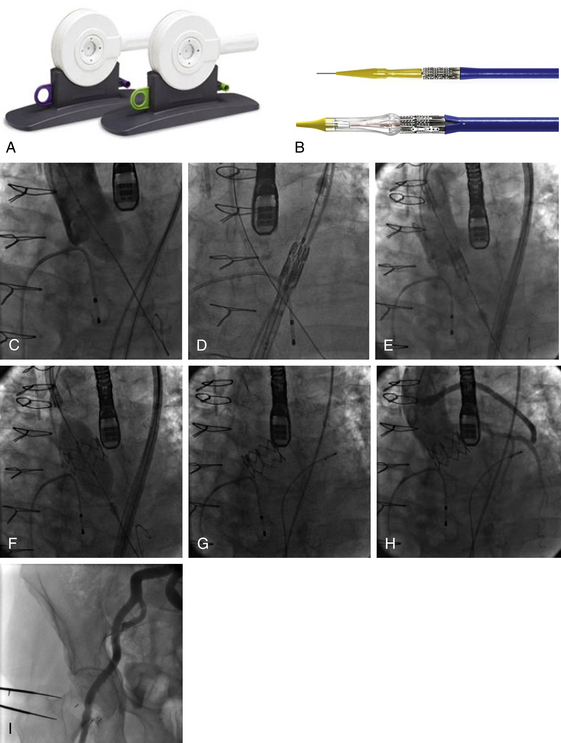
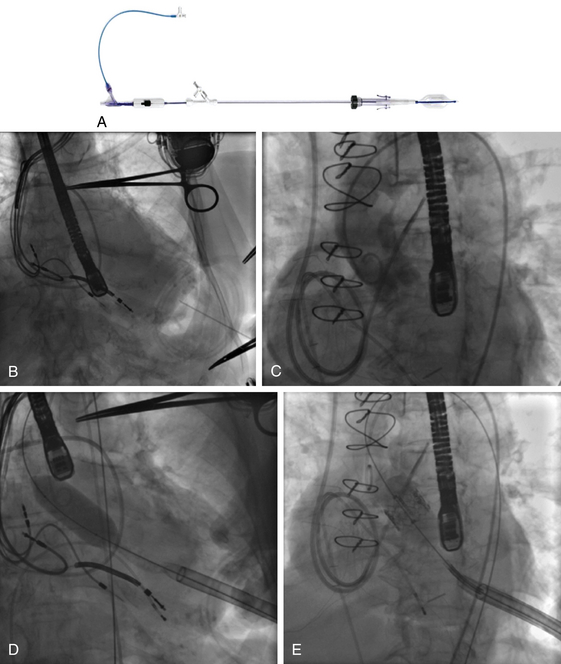
Chapter 6 Aortic stenosis (AS) remains the most common form of adult acquired valvular heart disease in developed countries, increasing in prevalence with age.1 As noted by Ross and Braunwald,2 the natural history of symptomatic AS carries a poor prognosis. Medically treated patients with symptomatic AS have 1 and 5 year survival rates of 60% and 32% respectively.3 Aortic valve replacement (AVR) is the only effective treatment for symptomatic AS that alleviates symptoms and improves survival. In the ideal candidate, surgical AVR has an estimated operative mortality of 4%.4 However, the operative mortality and incidence of postoperative complications increases with age, becomes significantly higher when surgery is done urgently, and when preexistent comorbidities such as coronary artery disease (CAD), poor left ventricular function, renal insufficiency, pulmonary disease, and diabetes are present.5,6 These factors are considered to be the main reasons that one third of patients with valve disease are not referred for surgery.7 Transcatheter aortic valve replacement (TAVR) has opened the possibility for treating patients who have been left untreated because it was believed that their operative mortality outweighed the benefits offered by traditional AVR. The concept of catheter-based valve therapies dates to the 1970s with the development of experimental approaches for the treatment of aortic insufficiency (AI).8–10 In 1989 Henning-Rud Andersen11 first implanted an original model of a balloon-expandable, catheter-mounted stented valve within the aortas of pigs, using a handmade mesh containing a porcine valve. Andersen’s and others’ experimental concepts emerged in the early 1990s but were not followed with human application.11–14 In 2000 Philip Bonhoeffer developed a stented valve made of a bovine jugular vein conduit inserted in a platinum-iridium stent, which was implanted in the pulmonary arteries of lambs.15 Bonhoeffer performed the first human implantation of this device in a right ventricle–to–pulmonary artery conduit in 2000,16 marking the beginning of the era of transcatheter valve replacement. TAVR was introduced in 2002 by Dr. Alain Cribier17 and utilized a bioprosthetic valve made of equine pericardium mounted on a balloon-expandable stainless steel stent. The first procedure was done via an antegrade approach in a subset of patients with severe AS who were not surgical candidates for reasons of compassion.17–20 Moderate to severe aortic insufficiency, valve embolization, and procedural complexity limited diffusion of the procedure. Significant technical and prosthetic modifications solved the previously encountered limitations. To reduce the degree of perivalvular regurgitation, valves were oversized in relation to the aortic annulus, and a second prosthesis size, 26 mm, became available. The transverse diameter of the aortic annulus at the level of aortic leaflet insertion was identified for appropriate valve sizing. In addition the necessary landmarks for valve positioning were recognized, decreasing the risk of valve embolization. A catheter with a manually activated deflectable tip (Retroflex catheter; Edwards LifeSciences, Irvine, Calif.) that aids in the atraumatic passage across the aortic arch and in centering the guidewire through the aortic commissures facilitated the valve delivery through the retrograde approach. Modifications in the delivery sheath also reduced vascular complications. Sheath length was increased to deliver the catheter/valve ensemble directly in the descending aorta, decreasing the risk of vascular injury.21 Multicenter registries from the United States (REVIVAL II), the European Union (REVIVE II), and Canada (Canadian Special Access) included patients with a valve area less than 0.8 cm2 and a high predicted operative mortality (logistic European System for Cardiac Operative Risk Evaluation [EuroSCORE] >20%) to continue to evaluate procedural safety and efficacy. New valve modifications were added to improve long-term function; these included use of bovine pericardium, elongation of the skirt to decrease perivalvular insufficiency, and the addition of an anticalcification treatment, culminating in the prosthesis that is currently used (Figure 6–1). The series of retrograde implantation published by Webb et al21 showed initial procedural success of 78%, which increased to 96% after the first 25 cases, reflecting an important learning curve.22 Observed 30-day mortality was 12%, and the expected 30-day mortality was 28%. At median follow-up examination, there was no evidence of valve deterioration, migration, restenosis, or central valvular insufficiency. Moderate perivalvular leaks were seen in three cases at 1 month. Perivalvular AI was mild, clinically inconsequential, and stable during follow-up study in the majority of the patients. This information led to the approval and valve commercialization in Europe in the fall of 2007. Figure 6–1 A, The Edwards SAPIEN valve is a trileaflet bovine pericardial valve mounted on a balloon-expandable stainless steel stent that is placed in the subcoronary position. A positron emission tomography (PET) skirt is placed around the ventricular third of the valve to decrease perivalvular insufficiency. B, The second generation SAPIEN XT valve has a new bovine pericardial leaflet design, is mounted on a cobalt chromium stent that reduces its profile, and has a longer skirt to minimize perivalvular insufficiency. Minimal arterial diameter, vessel tortuosity, and vessel calcification were still the major limiting factors. For these patients the transapical (TA) approach was envisioned. Initial experience in an animal model has been extrapolated to early human experience with promising results.23,24 The first published data related to the TA approach was from Lichtenstein et al23 and consisted of 7 high-risk patients with AS. Valve implantation was successful in all with no procedural deaths. Transvalvular gradient and aortic valve area improvement was seen in all patients, and the results were consistent with those found after retrograde implantation. Observed 30-day mortality was lower than the expected mortality (14% vs. 35%).25 Published multicenter experience using this innovative approach has been growing. Walther et al.26 reported 93.2% successful implantations with a conversion rate to traditional AVR of 6.8%. Patients are considered to have a high operative risk when their projected mortality is in the upper decile or have a 30-day mortality greater than 15%. Surgical risk is most commonly estimated by the Society of Thoracic Surgery Predicted Risk of Mortality (STS-PROM) and the EuroSCORE. The EuroSCORE has been validated in patients undergoing valvular surgery.27,28 However, this algorithm has been shown to persistently overestimate the mortality rate.29,30 The STS-PROM score is derived from the STS database, a voluntary registry of practice outcomes, and estimates the risk of mortality, morbidity, renal failure, and length of stay after valvular and nonvalvular cardiac surgery.31 This score has been shown to underestimate the true mortality rate after cardiac surgery, but it more closely reflects the operative and 30-day mortality for the patients having AVR who are at the highest risk.32 The STS-PROM and the EuroSCORE provide an objective way to quantify risk. Although thorough, these risk scores do not include certain characteristics that would complicate surgery and increase the operative mortality such as previous mediastinal irradiation, the presence of a severe calcification in the thoracic aorta (porcelain aorta), anatomic abnormality of the chest wall, history of mediastinitis, liver cirrhosis, or patient’s frailty. In addition, the algorithms were calculated from patients who underwent surgery, thus limiting their applicability to patients who were not considered surgical candidates. Clinical judgment and the patient’s level of independent function are subjective parameters that influence outcomes after cardiac surgery but are difficult to measure.33 Judicious use of TAVR is recommended in order to maximize appropriate outcomes. The evaluation of all patients undergoing TAVR should include: 1. A screening echocardiogram to document the severity of AS, verify the absence of other severe valvular disease, describe the valve anatomy and calcium distribution, and determine aortic annular diameter and left ventricular function. The presence of a protuberant septum at the level of the left ventricular outflow tract is important to note, because its presence may impede appropriate valve deployment. 2. Right and left catheterization to determine the presence of pulmonary hypertension and concomitant CAD, which may need to be treated before valve implantation. 3. Aortic angiography to note the correct orientation of the image intensifier during valve positioning and to determine potential complicating factors in the aortic arch that may interfere with the procedure. 4. Thoracoabdominal computed tomography (CT) angiography with iliofemoral runoff to determine the anatomy of the aorta, the vessel diameter, calcification, and tortuosity. After the screening process has been completed and the patients have been deemed eligible for TAVR, the route of implantation needs to be determined. There are two implantation routes available for the Edwards SAPIEN (Edwards Lifesciences, Irvine, Calif.) valve: (1) TA and (2) TF. Both delivery methods are comparable in success and complication rates. Selection depends on tortuosity, calcification, and internal diameter of the femoral, external iliac, and common iliac arteries. The presence of abdominal aortic aneurysms or history of their repair would favor the use of a TA or subclavian approach. Vascular complications have been associated with significant mortality and may be prevented with appropriate screening.34 Safety should not be sacrificed if both approaches are available and patients are considered good candidates. Contrast angiography provides an appropriate screening tool for route selection35; however, it does not provide a detailed determination of the vascular anatomy. The presence of an outer sheath diameter–to–femoral artery minimal luminal diameter ratio of 1.05 or greater is a predictor of major vascular complications and 30-day mortality.36,37 By inserting a guidewire across the iliac arteries, one can evaluate the degree that the vessels will straighten; if the arteries persist with significant tortuosities after insertion of a stiff guidewire, a TA approach is preferred. Multislice computed tomographic angiography (MSCTA) allows for precise determination of the degree, extent, and localization of vascular calcification. In addition, three-dimensional vessel reconstruction and cross-sectional imaging allow for precise determination of vessel luminal diameter. The minimal luminal diameter and the length of the segment with the minimal luminal diameter are the main considerations for selecting the delivery approach. Contrasted MSCTA is preferred; however, if chronic renal insufficiency precludes the use of a fully contrasted study, then intraarterial administration of a small contrast bolus38 or a noncontrasted CT may provide appropriate images for the necessary measurements. The use of intravascular ultrasound provides an invasive way of measuring the arterial diameter; however, it may provide unreliable dimensions because of image obliquity. The first generation Edwards SAPIEN valve requires minimal luminal diameters of 7 and 8 mm to allow for the 22F and 24F sheaths that are required for valve delivery. The new Edwards SAPIEN XT valve allows for use in vessels of 6 and 6.5 mm, requiring 18F and 19F sheaths, respectively (Table 6–1). Special attention needs to be given at the level of the aortoiliac and internal–external iliac artery bifurcations, because these areas tend to be involved in vascular complications. The aortic annulus diameter can be measured with different imaging modalities: transthoracic echocardiogram (TTE), transesophageal echocardiogram (TEE), contrast angiography, multislice computed tomography (MSCT), or magnetic resonance imaging (MRI). The diameter is measured in midsystole at the level of leaflet insertion. Because of the lack of a sewing ring, catheter-based prostheses need to be oversized by 5% to 30% relative to the aortic annulus in order to ensure valve stability and diminish AI. The aortic annulus appears to be noncircular, which may lead to underestimation by planar imaging. MSCT provides coronal, sagittal, and axial images of the annulus and aortic root, allowing the determination of the minimal and maximal diameter, annular circumference, and area.39,40 Annular determinations by TTE or TEE appear to be most commonly obtained. However, as the annular determination becomes more uniform, it is expected that MSCT will be the primary sizing modality (Table 6–2). Patients with concomitant CAD have higher operative mortality risk scores and associated comorbidities than those without CAD. It is recommended to revascularize the vessels that supply a large area of myocardium at risk while disregarding the smaller branches before TAVR.41–43 The timing of revascularization is controversial. Patients enrolled in the PARTNER trial required a minimum of 30 days between coronary revascularization and valve implant. Revascularization is generally preferred before TAVR if the amount of myocardium at risk is large. It can be done in the same setting before TAVR or a couple of days earlier in order to minimize radiation and contrast exposure and other potential complications. The Edwards SAPIEN Valve (see Figure 6–1) is a trileaflet bioprosthesis made of bovine pericardium that is mounted in a balloon-expandable stainless steel stent. It has been pretreated to decrease calcification and functional deterioration. The stent has a fabric cuff placed in the ventricular side that covers one half of the frame, limiting stent expansion and decreasing perivalvular insufficiency. Because of the lack of a sewing ring, the valve is oversized to the aortic annulus to ensure postdeployment stability and is currently available in two sizes: 23 mm and 26 mm. In bench-top testing, its durability is greater than 10 years. The SAPIEN valve provides a larger effective orifice area and lower hemodynamic profile than corresponding surgically implanted valves but have a higher incidence of perivalvular insufficiency.44 The new generation device, the Edwards SAPIEN XT valve, is commercialized in Europe and made of a cobalt–chromium alloy that provides the same radial strength yet reduces the valve profile. This valve is available in 20-mm, 23-mm, 26-mm, and 29-mm sizes. The ongoing PARTNER II Trial is designed to evaluate this valve technology (see Figure 6–1). The valve is mounted on a custom made balloon that is 30 mm in length with balloon diameters that correspond to the prosthesis size. The balloon ends in a nose cone that facilitates crossing the native valve (Figure 6–2). Its inflation profile decreases movement during inflation. Figure 6–2 For the transfemoral transcatheter aortic valve replacement (TAVR) (A), the SAPIEN valve is crimped over the delivery catheter (B). Supraaortic angiography is performed in a view that places all three coronary cusps in the same plane. C, A balloon aortic valvuloplasty (BAV) is performed to facilitate valve positioning. The delivery catheter is advanced through the descending aorta (D). The retroflex catheter is activated to allow the passage of the valve through the aortic arch. Correct valve position is confirmed by echo and angiography (E), the valve is deployed during rapid ventricular pacing (RVP) (F). Aortic angiography confirms valve position, lack of aortic insufficiency, and unrestricted flow through native coronary arteries and bypass grafts (G and H). The delivery sheath is removed and iliofemoral angiography is performed to confirm the absence of vascular complications (I). A crimping tool (see Figure 6–2) is used to manually and symmetrically compress the overall diameter of the transcatheter heart valve (THV) from its expanded size to its minimal delivery profile. A measuring ring is used to calibrate the balloon inflation to its desired size and to determine the volume of saline/contrast mixture in the syringe necessary for the proper inflation at the time of deployment. The Retroflex catheter has a deflectable tip that changes direction when activated by the rotation of an actuator incorporated in the handle, facilitating the passage of the THV across the aortic arch from the retrograde approach45 (see Figure 6–2). The catheter is used to direct the valve delivery system through the arterial system, around the aortic arch, and across the aortic valve, providing a less traumatic passage. The Retroflex catheter assists in centering and supporting the valve as it crosses the calcified and stenotic native valve. This system also provides precise positioning at the aortic annulus. The Novoflex catheter (Edwards Lifesciences, Irvine, Calif.) is a newer generation catheter that allows loading the Edwards SAPIEN XT prosthesis onto the balloon while it is in the body, decreasing the sheath size dramatically. The Ascendra delivery system (Edwards LifeSciences, Irvine, Calif.) is the delivery catheter used for the TS route (Figure 6–3). This catheter allows for easy valve manipulation to improve the prosthetic orientation. Figure 6–3 Transapical delivery of the SAPIEN valve requires the Ascendra delivery system (A). The procedure starts with a lateral thoracotomy. After the heart is exposed and a purse string suture is placed around the puncture site, a needle is placed in the left ventricle (LV) apex (B) to mark the trajectory. C, An aortic angiogram is performed to align the aortic cusps in the same plane, and the delivery sheath is inserted into the LV cavity. D, A balloon valvuloplasty is performed through the delivery sheath during rapid ventricular pacing (RVP). E, The valve is then advanced to its appropriate position and deployed during RVP. The THV assembly and deflecting guiding catheter are introduced through a 25-cm hydrophilic coated sheath that extends into the abdominal aorta to decrease vascular complications. The TF delivery system for the SAPIEN valve requires 22F and 24F sheaths for the 23- and 26-mm valves, respectively. With the introduction of SAPIEN XT and Novoflex, the insertion sheath has decreased to 18F and 19F, respectively. The sheath is equipped with a hemostatic mechanism to decrease blood loss. For the 23-mm THV, the introducer sheath has an outer diameter of 7.2 mm (21.6F) and an inner diameter of 6 mm (18F). For the 26-mm THV, the introducer sheath has an outer diameter of 7.5 mm (22.5F) and inner diameter of 6.3 mm (19F). Other improvements in the access sheath include the development of the eSheath (Edwards Lifescience, Irvine, Calif.). The eSheath has a dynamic expansion mechanism that allows the sheath to transiently expand as the Novoflex delivery system is pushed forward and then recoil to its nominal diameter. The eSheath has an inner diameter of 5.3 mm (16F) and outer diameter of 6.6 mm (20F) for implantation of the 23-mm THV and an inner diameter of 6.0 mm (18F) and outer diameter of 7.2 mm (21 to 22F) for implantation of the 26-mm THV. TF–TAVR can be performed in the cardiac catheterization lab or in the hybrid operating room, provided that the room is equipped with a fixed fluoroscopy unit that provides high image quality and the ability to store reference images for road-mapping. There must be enough room for operators to work comfortably and the circulators to move freely. A cardiopulmonary bypass machine should be accessible if complications arise. Equipment to treat peripheral vascular or coronary complications should be stocked in the room and available on demand (see Figure 6–2). The procedure can be done when the patient is under general anesthesia46,47 or conscious sedation. General anesthesia is preferred if a TEE is performed simultaneously during the procedure. If not, conscious sedation and local anesthesia may provide enough relief during the procedure. Continuous invasive hemodynamic monitoring should be used throughout the procedure.
Transcatheter Aortic Valve Replacement
Edwards SAPIEN Valve
6.1 Patient Selection for Transcatheter Aortic Valve Replacement
Risk Stratification
6.2 Patient Screening
Vascular Screening
Aortic Annulus Diameter and Prosthetic Sizing
Coronary Disease
6.3 The Edwards SAPIEN Valve
The Edwards Sapien Valve
The Balloon Catheter
The Crimping Tool
The Retroflex Guiding Catheter and the Ascendra Delivery System
Delivery Sheath
6.4 Transfemoral Valve Implantation
Room Requirements
Anesthesia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine