Transcatheter Aortic Valve Replacement
Eric L. Sarin
Vinod H. Thourani
Introduction
Surgery to replace a dysfunctional aortic valve remains the most common indication for valvular heart surgery. The increased incidence of degenerative aortic valve disease among the elderly coupled with increasing life expectancies across the Western world has resulted in a growing number of older patients with clinically significant aortic valve disease. As a result, surgeons are seeing older patients, with more comorbidities, who are at an increased risk for perioperative morbidity and mortality.
In the past, a significant subset of these patients was not referred for surgical evaluation because of their perceived risk of complications. The rise of transcatheter aortic valve replacement (TAVR) over the last decade has offered promise as a durable therapeutic option for those patients previously considered to be at increased risk or unsuitable for surgical valve replacement with cardiopulmonary bypass (SAVR).
The indications for TAVR in those with aortic stenosis (AS) are similar to those for surgical aortic valve replacement. Symptomatic patients with severe aortic stenosis, defined as a peak aortic jet velocity (Vmax) ≥4 m/s, aortic valve area (AVA) <1 cm2, or mean aortic valve gradient ≥40 mm Hg, can be considered for TAVR. Currently in the United States, TAVR is commercially approved for patients with greater than 1-year life expectancy and symptomatic, severe AS who are considered to be either at high-risk for perioperative complications or inoperable. This decision should be made by a multidisciplinary Heart Team representing specialists in cardiac surgery, interventional cardiology, echocardiography, and cardiac anesthesia. High-risk patients are defined by a Society of Thoracic Surgeons’ (STS’) predicted risk score of ≥8% or by comorbid conditions that are associated with a predicted risk of perioperative death ≥15%.
Patient selection is of paramount importance when planning a TAVR. In addition to the standard preoperative evaluation for comorbid disease, a thorough TAVR-specific pre-op evaluation should address the following:
Severity of aortic stenosis, including the anatomic details of the valve leaflets (leaflet height, bicuspid vs. tricuspid)
Peripheral vascular disease: ileofemoral vessel size, calcification, and tortuosity, and prior surgical procedures (iliac stenting, aortoiliac reconstruction)
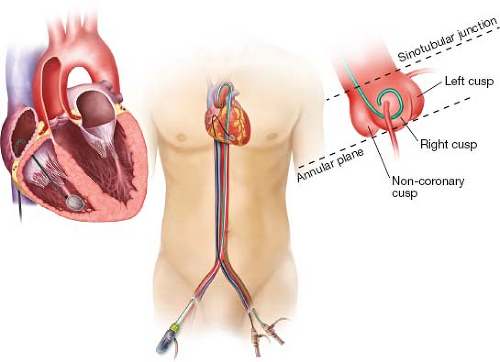
Annular, sinotubular, and sinus of Valsalva dimensions
Ventricular function and concomitant valvular pathology (e.g., severe mitral or tricuspid regurgitation)
Presence of concomitant coronary artery disease
Typically, a combination of imaging techniques is used to answer these questions. Our institution routinely employs left heart catheterization; high-definition computed tomography (CT) of the chest, abdomen, and pelvis; and transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE). Furthermore, objective frailty testing, pulmonary function tests (PFTs), and carotid duplex ultrasound are performed to assess lung function and carotid stenosis. Hemodynamically significant and symptomatic carotid lesions should be treated prior to TAVR.
Aortic Valve Assessment
All patients should have a CT scan from the aortic valve to the femoral bifurcation. A gated, contrast CT with 3-D reconstruction provides detailed information regarding the aortic root anatomy, particularly dimensions of the aortic annulus, sinotubular junction, and sinuses of Valsalva. Also of considerable importance are the heights of the coronary ostia above the annular plane. Measurement of the annular area or perimeter has largely supplanted two-dimensional echocardiography and is considered the standard of care for TAVR sizing. Each TAVR valve provides a sizing chart corresponding to the annular area or perimeter.
A complete echocardiographic assessment, typically a TTE, is required for each patient and provides complimentary information to the CT scan. From the parasternal long-axis view, the right and noncoronary cusps are identified, and the annulus is measured between the insertion points of the leaflets. Accurate measurement is essential as it will be used to choose the size of the bioprosthesis. If calcification, body habitus, or other factors preclude the accurate measurement of the annulus, a TEE should be performed.
Some patients may end up in-between valve sizes based on their CT scan and echocardiographic data. In these instances, balloon sizing of the annulus with supravalvular aortography during balloon aortic valvuloplasty (BAV) may provide additional information to guide prosthesis sizing. The surgeon must bear in mind that based on BAV sizing, if a larger valve is chosen this may increase the delivery sheath size. In this instance, a thorough knowledge of the ileofemoral vessel dimensions is critical.
Lower Extremity Assessment
Assessment of the lower extremities begins with either a CT scan or lower extremity angiography at the time of cardiac catheterization. Particular attention must be paid to the vessel diameters of the ileofemoral system bilaterally. Significant calcification of the ileofemoral system is not uncommon, but does not necessarily preclude a femoral artery approach. However, in cases of severe calcification where the involvement is circumferential and size-limiting, alternative access sites should be considered.
Tortuosity, particularly in the external iliac artery, may limit the safe advancement of the delivery sheath. Even moderate calcification of a tortuous external iliac artery can increase the likelihood of major vascular complications. Tortuous vessels that are
free of significant calcification are often compliant and can usually be straightened by a stiff guidewire. While previous surgical procedures (aortoiliac replacement, iliac stenting) are not absolute contraindications to a femoral approach, they need to be approached with caution. Synthetic graft material will not behave like native tissue and may prove difficult to traverse with the necessary sheaths and guidewires. This should always be done under direct fluoroscopic visualization with a low threshold for conversion to alternative access if significant resistance is met.
free of significant calcification are often compliant and can usually be straightened by a stiff guidewire. While previous surgical procedures (aortoiliac replacement, iliac stenting) are not absolute contraindications to a femoral approach, they need to be approached with caution. Synthetic graft material will not behave like native tissue and may prove difficult to traverse with the necessary sheaths and guidewires. This should always be done under direct fluoroscopic visualization with a low threshold for conversion to alternative access if significant resistance is met.
Coronary Considerations
Patients with severe coronary artery disease and significant lesions that are amenable to percutaneous coronary intervention (PCI) should undergo implantation utilizing a bare metal or drug-eluting stent prior to TAVR, particularly if rapid pacing is planned during the procedure. The transient hemodynamic instability often seen during TAVR deployment will be magnified in patients with an increased coronary ischemic burden.
Another potential concern that may be raised during preoperative evaluation is the risk of acute coronary obstruction following valve deployment. A narrow sinus of Valsalva (within 5 mm of the annulus size), short coronary ostia height (<10 mm), and bulky leaflet calcification may all increase the risk for coronary obstruction. These patients should have guidewires or an angioplasty balloon placed in the affected coronary ostia prior to TAVR deployment to facilitate rapid stenting, if necessary.
Alternative Access Considerations
If the preoperative evaluation determines that TF-TAVR is not feasible, alternative access techniques should be considered. The transapical (TA) approach, done via an anterior left mini-thoracotomy, was the main alternative used in the PARTNER trials. In experienced hands this approach is very effective, but may not be appropriate for all patients, particularly those with significant parenchymal lung disease or low ejection fraction. The subclavian and transaortic (TAo) approaches were popularized by the CoreValve trial and are gaining in popularity. Far less common approaches for those patients who are not suitable for TA, TAo, or subclavian are the transcarotid and transcaval approaches.
Transfemoral TAVR
Transfemoral delivery is the least invasive TAVR approach and has become the procedure of choice in patients with appropriate vasculature. A 6-Fr sheath is placed in the artery and vein on the nonimplant side. A pigtail catheter is advanced to the aortic valve and aortography is performed to confirm the correct valve plane for valve placement. Typically, this is performed with the pigtail catheter in the right coronary cusp. The proper deployment angle is identified when all three cusps are aligned at an equal height (Fig. 2.1).
Using the femoral vein, a temporary pacemaker is advanced to the right ventricular apex for purposes of rapid ventricular pacing. The ability to provide consistent pacing capture is critical, particularly for balloon-expandable TAVR deployment. In order to gain stability and facilitate adequate contact of the pacemaker with the right ventricular myocardium, we utilize an 8-Fr Mullins sheath advanced into the right atrium.
The femoral artery on the implant side can be accessed by direct surgical cutdown or percutaneously depending on the surgeon’s preference. Percutaneous access to the femoral artery is performed utilizing a microneedle under vascular road mapping to assure puncture of the anterior wall of the vessel. Once wire access is confirmed, a 7-Fr
sheath is inserted and removed to dilate the tract and the artery is preclosed with two Perclose devices. Over an extra-stiff wire, the vessel is serially dilated to accommodate the delivery sheath. The patient is heparinized to an ACT of >250 seconds. Excessive force should not be applied when passing the dilators to avoid vascular complications during insertion and removal.
sheath is inserted and removed to dilate the tract and the artery is preclosed with two Perclose devices. Over an extra-stiff wire, the vessel is serially dilated to accommodate the delivery sheath. The patient is heparinized to an ACT of >250 seconds. Excessive force should not be applied when passing the dilators to avoid vascular complications during insertion and removal.
Next, the aortic valve is crossed using an Amplatz left-1 (AL1) catheter and a straight guidewire. This is then exchanged for a 260-cm long, 0.035-inch Amplatz extra-stiff J-tipped wire with an exaggerated pigtail bend at the proximal end. In those undergoing a self-expanding valve implantation, a super-stiff J-tipped wire can be inserted in the left ventricle in lieu of the extra-stiff wire. BAV is performed under rapid ventricular pacing (generally 180 to 220 beats/minute) with an appropriately sized balloon catheter.
The appropriate valve delivery system is inserted and advanced to the aortic annulus. Difficulty in crossing the aortic valve can occur as the stiff wire may bias to the greater aortic curvature and may become lodged in a commissure. Gentle traction on the wire will center the delivery system and facilitate crossing the valve. Once across the native valve, the system should not be advanced further to avoid perforation of the left ventricular apex. Proper positioning of the valve is guided by fluoroscopy and echocardiography.
The SAPIEN valve is ideally positioned so that its upper margin covers the aortic leaflet tips, while the ventricular end covers the aortic annulus or below. The CoreValve’s position is determined by its three-tiered design, with the inflow portion of the valve ideally placed 6 mm (one diamond segment of the stent frame) below the point of leaflet attachment. This places the portion of the valve with the highest radial force firmly in the annulus, but not so low as to compress the adjacent cardiac conduction system.
When the device is appropriately positioned, the implanting physician should coordinate a long cine-fluoroscopic run. For balloon-expandable valves, we advocate a slow, controlled inflation to ensure proper valve position. Maximal inflation should be held for 3 to 4 seconds before deflation. It is important that pacing begins before balloon inflation and continues uninterrupted until the balloon is near complete deflation. Successful rapid pacing with 1:1 ventricular capture at 180 to 220 bpm will lower the blood
pressure below 60 mm Hg, and prevents forceful contractions that may lead to inadvertent ejection of the valve prosthesis.
pressure below 60 mm Hg, and prevents forceful contractions that may lead to inadvertent ejection of the valve prosthesis.
Self-expanding valves, such as the CoreValve system, do not commonly require rapid ventricular pacing during deployment. Pacing at a lower rate (100 to 120 bpm) has been advocated by many practitioners to mildly lower the blood pressure during the final stages of valve deployment. The CoreValve has the benefit of becoming functional before the valve is completely deployed. This allows for aortography and assessment of valve function while a degree of repositionability still exists.
Following deployment of the valve, positioning and function is assessed by echocardiography and aortography. A trace or mild amount of paravalvular leak is expected after TAVR. If there is more than mild leak around a correctly positioned valve, postdeployment balloon dilation using the balloon on the delivery catheter may further expand the valve and improve the insufficiency.
Transapical TAVR
The TA-TAVR technique was the primary alternative access site for the PARTNER trial and has so far been used predominantly with a balloon-expandable valve. In those patients with severe peripheral vascular disease, the TA approach is a very expeditious procedure. This technique represents the only antegrade approach that is regularly employed. We particularly favor a TA approach in those with a prior sternotomy or porcelain aorta. The most feared complication of this approach is bleeding from the left ventricular cannulation site, but this complication is uncommon and decreases with operator experience. The only relative contraindications to the TA procedure are severe COPD with an FEV1 %predicted <30% or an ejection fraction <20%.
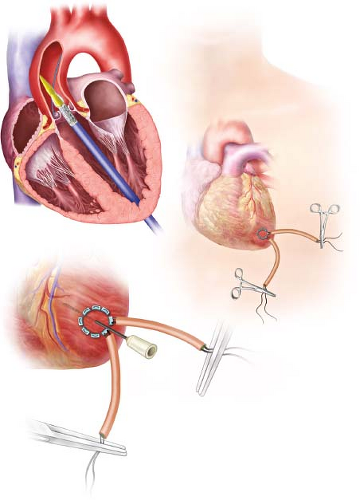
The patient is placed supine on the operating room table and femoral arterial and venous access is obtained as previously described. A femoral transvenous pacer is placed in the right ventricle and a pigtail catheter is placed in the aortic root via the femoral artery. Direct AP fluoroscopy is used to localize the LV apex and a 4- to 5-cm anterolateral thoracotomy is then made in the fifth or sixth intercostal space. Once the LV apex is exposed, intravenous lidocaine is administered and two pledgeted 3-0 prolene pursestring sutures are placed just cephalad to the true apex and lateral to the LAD. The pursestring sutures in the myocardium should be deep stitches, but not transmural to avoid tearing (Fig. 2.2).
The patient is heparinized and the left ventricular cavity is accessed with a needle and a 0.035-inch wire. Using fluoroscopy, the wire is passed into the left ventricle, across the aortic valve, and into the ascending aorta. The wire is maintained in the ascending aorta and not allowed to pass into the right carotid artery to prevent a cerebrovascular accident. A 7-Fr catheter is placed through the left ventricular apex and across the aortic valve. The 0.035-inch wire is manipulated into the descending aorta using a right Judkins catheter. The 0.035-inch wire is exchanged for a super-stiff wire (Amplatz super stiff; Boston Scientific, Natick, MA) and left in the abdominal aorta. The 7-Fr catheter is exchanged for the appropriate-sized delivery sheath which is positioned 3 to 4 cm inside the LV. BAV can be performed with or without rapid ventricular pacing, but because the valve is crossed in antegrade fashion, predilation is not always necessary. The balloon is removed and the valve is placed through the LV delivery sheath and positioned across the valve. Except for cases that require balloon sizing for choice of valve prosthesis, we do not commonly perform a BAV in our TA-TAVR cases. Positioning, deployment, and postassessment of the valve is similar to that as aforementioned in the TF-TAVR section. The TA approach offers the surgeon exquisite control of valve position and oftentimes, only subtle movements are required during deployment. After adequate evaluation, all catheters and wires are removed and the apical sutures are tied down using rapid ventricular pacing. Protamine is administered. There can be no tolerance for bleeding or oozing of any kind from the cannulation site and any concerns regarding hemostasis must be addressed prior to chest closure. Once hemostasis has
been assured, a small drain is placed in the left pleural space and the incision is closed in the standard fashion.
been assured, a small drain is placed in the left pleural space and the incision is closed in the standard fashion.
Transaortic TAVR
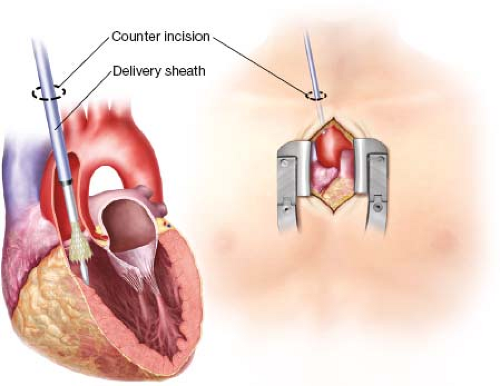
The TAo approach is gaining increasing popularity as an alternative access site for both balloon- and self-expanding valves. The TAo approach is advantageous because it avoids a thoracotomy in patients with poor respiratory function and may be less painful than the TA approach. Accessing the aorta via an upper mini-sternotomy incision is a standard approach for minimally invasive valve surgery. Furthermore, direct aortic cannulation is a procedure that all cardiac surgeons feel comfortable with, particularly when compared to TA access which is less commonly used.
However, while the TAo approach offers several advantages, it is not appropriate for all patients. The TAo approach is contraindicated in patients with a heavily calcified or “porcelain” aorta or in an otherwise hostile mediastinum (i.e., patients with a history of cobalt radiation). It can also be technically challenging in the setting of a prior sternotomy, especially if the patient has patent grafts from a previous coronary artery bypass grafting (CABG). Particular attention should be paid to patients with a prior CABG using a pedicled right internal mammary artery graft. If the artery was brought across midline anterior to the aorta it can be very easily injured and we would not
recommend the TAo approach in this setting. Thorough review of the preoperative CT scan will help mitigate these potential complications.
recommend the TAo approach in this setting. Thorough review of the preoperative CT scan will help mitigate these potential complications.
 Get Clinical Tree app for offline access 
|