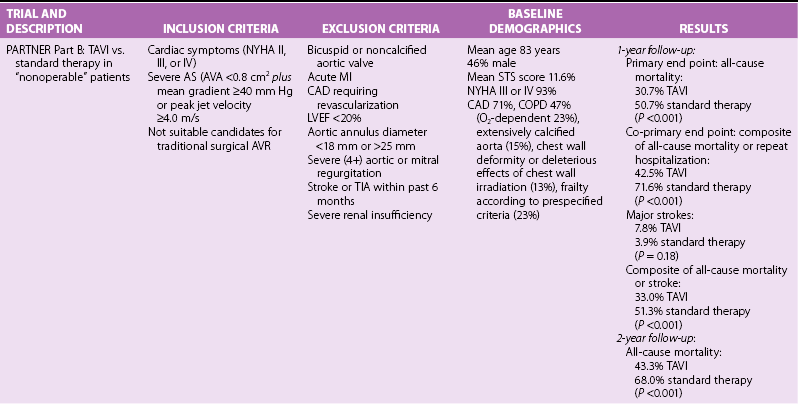
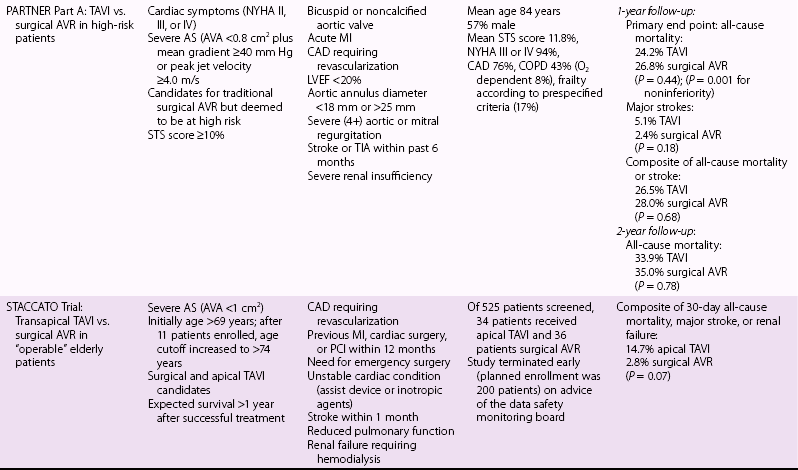
Chapter 15 The outlook for patients with symptomatic valvular aortic stenosis (AS) is grave, with death rates of 51% at 1 year and 68% at 2 years in one series of patients who were deemed not to be surgical candidates and were treated with standard medical therapy (including balloon aortic valvuloplasty). 1 Definitive therapy of valvular AS requires relief of obstruction to left ventricular ejection. Currently, open surgical aortic valve replacement (or repair in rare instances) (AVR) is the most commonly used modality and offers excellent long-term results. 2 However, given the aging of the population, the frequency of patients presenting for reoperation, and medical advances that have allowed patients with comorbidities to survive and present with valvular AS, a significant number of patients are deemed to have excessive risk for traditional surgery. 3 Percutaneous techniques, therefore, were developed initially to offer relief of obstruction to left ventricular ejection in patients who were not candidates for surgery. Initial results with balloon aortic valvuloplasty were disappointing, with neither hemodynamic nor clinical improvement over the long term. 4 The need for alternative treatment options, including transcatheter aortic valve implantation (TAVI), is demonstrated by the Euro Heart Survey on Valvular Heart Disease, in which up to one third of patients with symptomatic AS were denied traditional surgery.3,5 Interest in TAVI started with work of Andersen et al 6 in animals, followed by the work of a number of groups. Cribier et al 7 are credited with the first implantation in humans. Webb et al 8 popularized the retrograde arterial approach (initially from the femoral artery), which is now the standard for TAVI. These pioneers led the development of percutaneous techniques for aortic valve implantation and have begun a new era in the therapy of patients with valvular AS. TAVI is the only intervention for AS shown to prolong life in a randomized trial.1,9 In many experienced centers this procedure is now the standard of care for extremely high-risk or “inoperable” patients, and it is a valid alternative to surgery for many high-risk but “operable” patients. More than 100,000 TAVI procedures have been performed to date. In this chapter we present our current views on the valve designs, findings from randomized trials and registries, and implantation techniques for TAVI. Two types of aortic valves for percutaneous implantation have been used in a significant number of patients: balloon-expandable and self-expanding ( Figure 15-1). FIGURE 15-1 Current widely available transcatheter valves. Balloon-expandable prostheses for which there is extensive published data for human implantation include the first-generation Cribier-Edwards valve and the modified second-generation SAPIEN (SAPIEN and SAPIEN XT) series of valves (both from Edwards Lifesciences Corporation, Irvine, California) (see Figure 15-1). This state-of-the-art Edwards SAPIEN XT transcatheter heart valve is a balloon-expandable cobalt chromium alloy tubular frame within which are sewn bovine pericardium leaflets. The inflow of the frame is covered with a fabric cuff to provide an annular seal (see Figure 15-1). For transarterial implantation, the transcatheter valve is compressed onto a low-profile NovaFlex (Edwards Lifesciences) delivery catheter ( Figure 15-2) and introduced through a sheath placed in the femoral artery. Alternatively a sheath can be placed surgically in the left ventricular apex or ascending aorta. The SAPIEN XT valve is balloon-expanded within the diseased native valve, displacing the diseased native leaflets. A low-profile (16F to 19F) SAPIEN XT/NovaFlex transfemoral system is in widespread clinical use in many countries. The first Placement of AoRTic TraNscathetER Valve (PARTNER I) trial (see The PARTNER Trial) used the earlier SAPIEN valve, which requires the use of larger-diameter (22F to 24F) sheaths; this is the current system used clinically in the United States (see Figure 15-2). FIGURE 15-2 Valve delivery catheters. The self-expanding valve with the most published human implantation data is the CoreValve ReValving System (Medtronic, Inc, Minneapolis, Minnesota) (see Figure 15-1). Currently, 23-, 26-, 29-, and 31-mm devices allow treatment of patients with annulus diameters from 20 to 29 mm. The CoreValve technology features a multilevel frame with porcine pericardial leaflets. The term “frame” is used rather than “stent” because by engineering definition, a stent exhibits the same radial force at every point of its peripheral circumference; the CoreValve frame exhibits three entirely different radial and hoop strength levels at different parts of its peripheral circumference. The CoreValve is compressed within an Accutrak delivery catheter (Medtronic) (see Figure 15-2) and introduced through an 18F sheath into the common femoral or subclavian artery. Once the CoreValve is positioned correctly within the diseased native valve, the delivery catheter is withdrawn, releasing the valve. The multistage frame is anchored within the aortic annulus, but owing to its length also extends superiorly to anchor in the supracoronary aorta. The ACURATE TA (Symetis Inc., Ecublens, Switzerland) ( Figure 15-3) is a nitinol-based valve that incorporate features that facilitate positioning and anatomic orientation in relation to the native valve commissures and coronaries. The valve is currently implanted only transapically. The system received CE Mark approval on September 30, 2011, on the basis of studies conducted at 6 sites in Germany enrolling 90 high-risk patients with severe AS. 10 The mean logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation) was 20.4 ± 8.7% and the Society of Thoracic Surgeons (STS) risk score was 8.4 ± 6.4%. All patients had New York Heart Association (NYHA) functional class III or IV heart failure symptoms, the average age was 84 ± 4 years, and 69% were female. The procedural success rate was 94.4% (n = 85), with 2 patients requiring a valve-in-valve procedure and 3 patients requiring conversion to open surgery. FIGURE 15-3 Valves undergoing early evaluation. In an effort to reduce delivery catheter diameter, improve ease of positioning and sealing, or facilitate repositioning or removal, a number of newer transcatheter valves (see Figure 15-3) are in early clinical evaluation. Mostly nitinol based, the valves offer the following advantages: • Lotus valve (Boston Scientific Inc., Natick, Massachusetts) is designed to expand laterally as longitudinal nitinol wires are retracted • Direct Flow valve (Direct Flow Medical Inc., Santa Rosa, California) has a tubular fabric frame that is inflated with a rapidly setting polymerizing agent • ACURATE TA, already described, and Portico (St. Jude Medical, Inc., St. Paul, Minnesota) valves extend from the annulus to the supracoronary aorta to assist in coaxial alignment and fixation (like CoreValve) • Engager (Medtronic) and JenaValve (JenaValve Technology, Munich) valves incorporate features that facilitate positioning and anatomic orientation in relation to the native valve commissures and coronaries. The main 1- and 2-year results of the PARTNER trial1,9,11,12 are presented in Table 15-1. The following points deserve emphasis when one considers the results of the trial. The trial used early-generation TAVI systems (SAPIEN 23-mm and 26-mm valves with 22F and 24F femoral delivery catheters) in centers with minimal operator experience with TAVI. PARTNER part B compared transfemoral TAVI with standard therapy (including balloon aortic valvuloplasty). 9 At 1-year follow-up, the rates of death were 50.7% in the standard therapy group and 30.7% in the TAVI group; only five patients needed to be treated with TAVI to prevent one death at 1 year. TAVI was associated with a significant reduction in symptoms at 1 year as assessed by NYHA functional class (74.8% of surviving patients who had undergone TAVI were in NYHA class II or lower, versus 42.0% of those treated with standard therapy) and 6-minute walk test. The rate of major stroke was numerically (but not statistically) higher with TAVI, whereas those of vascular complications and major bleeding were significantly higher (see Table 15-1). The SAPIEN valve demonstrated good hemodynamic performance. In the 2-year follow-up of PARTNER part B, mortality remained lower in the TAVI group that in the standard therapy group (43.3% versus 68%), and the TAVI group had lower mortality between years 1 and 2 (18.2% mortality in patients alive at 1 year in the TAVI group versus 35.1% mortality in patients alive at 1 year in the standard therapy group). 1 It was noted that TAVI did not improve mortality in patients with an STS risk score higher than 14.9% upon entry into the trial. Rates of all strokes were higher in the TAVI group (13.8% versus 5.5%), the excess driven primarily by hemorrhagic events. PARTNER part A compared transfemoral and transapical TAVI with surgical AVR. 11 One-year data showed the non-inferiority of TAVI (death from all causes 24.2% with TAVI versus 26.8% with standard surgery). Of the 699 patients undergoing random allocation, 42 did not undergo the assigned procedure (4 in the TAVI group and 38 in the surgical group). Rates of major strokes were numerically (but not significantly) higher in the TAVI group. Subgroup analysis indicated that women and patients who had not undergone previous coronary artery bypass grafting (CABG) may benefit more from TAVI than from standard surgical AVR. The 2-year follow-up of PARTNER part A showed that the rates of death from any cause were not different between TAVI and surgical AVR (33.9% versus 35%), and the rate of stroke remained numerically (but not statistically significantly) higher in the TAVI group. 12 Multivariate predictors of mortality in the TAVI group included a higher body mass index (BMI) and higher preprocedure transvalvular gradient predicting lower risk, and reduced renal function and prior vascular surgery or stent predicting higher risk. Prior CABG was protective in the surgical group, whereas higher STS score, liver disease, or more than mild mitral regurgitation was detrimental. Stroke and major bleeding raised mortality in both groups.
Transcatheter Aortic Valve Implantation
Percutaneous Aortic Valve Designs
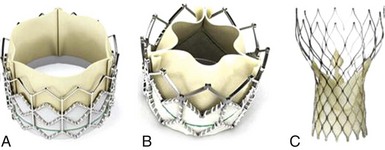
A, The Edwards SAPIEN THV balloon-expandable valve (Edwards Lifesciences Corporation, Irvine, California) incorporates a stainless steel frame, bovine pericardial leaflets, and a fabric sealing cuff. B, The SAPIEN XT THV (Edwards Lifesciences) utilizes a cobalt chromium alloy frame and is compatible with lower-profile delivery catheters. C, The Medtronic CoreValve (Medtronic, Inc, Minneapolis, Minnesota) incorporates a self-expandable frame, porcine pericardial leaflets, and a pericardial seal. (From Webb JG, Wood DA. Current status of transcatheter aortic valve replacement. J Am Coll Cardiol 2012;60:483–92.)
Balloon-Expandable Percutaneous Aortic Valves
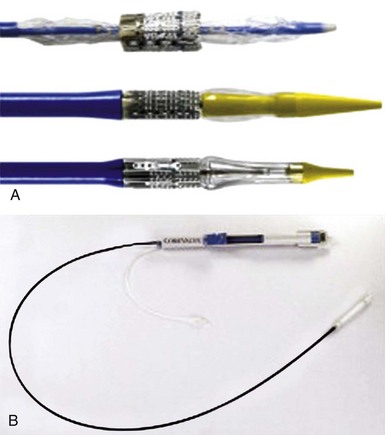
A, Top, the RetroFlex 1 delivery system for the Edwards SAPIEN THV (Edwards Lifesciences Corporation, Irvine, California) as used in the PARTNER 1 (Placement of AoRTic TraNscathetER Valve 1) trials (8-mm diameter). Middle, the RetroFlex 3 system (Edwards Lifesciences). Bottom, the NovaFlex/SAPIEN XT system (6-mm diameter; Edwards Lifesciences). B, The Accutrak delivery system with the Medtronic CoreValve (6-mm diameter, also with a tapered nose cone; Medtronic, Inc, Minneapolis, Minnesota). The prosthesis is enclosed within an outer sheath. (From Webb JG, Wood DA. Current status of transcatheter aortic valve replacement. J Am Coll Cardiol 2012;60:483–92.)
Self-Expanding Valves
“Next-Generation” Valves
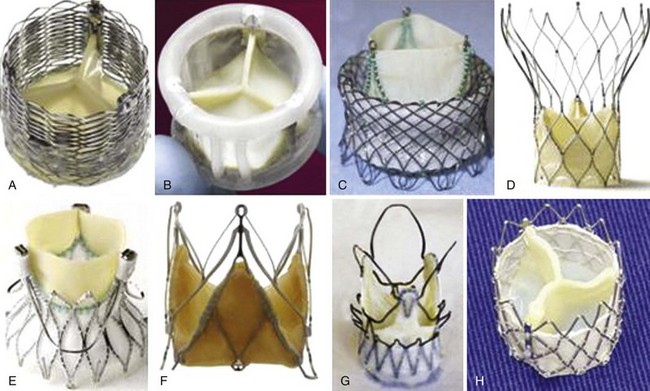
A, Lotus (Boston Scientific Inc., Natick, Massachusetts); B, Direct Flow (Direct Flow Medical Inc., Santa Rosa, California); C, HLT (Bracco Inc., Princeton, New Jersey); D, Portico (St. Jude Medical Inc., St. Paul, Minnesota), E, Engager (Medtronic, Inc, Minneapolis, Minnesota); F, JenaValve (JenaValve Technology, Munich); G, ACURATE TA(Symetis Inc., Ecublens, Switzerland); and H, Inovare (Braile Biomedica Inc., São José do Rio Preto, Brazil). (From Webb JG, Wood DA. Current status of transcatheter aortic valve replacement. J Am Coll Cardiol 2012;60:483–92.)
Randomized TAVI Trials
The PARTNER Trial
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree