50 Trans-catheter Aortic Valve Interventions
From Balloon Aortic Valvuloplasty to Trans-catheter Aortic Valve Implantation
Aortic stenosis (AS) remains the most common form of adult acquired valvular heart disease in developed countries, increasing in prevalence with age.1 As noted earlier by Ross and Braunwald, the natural history of symptomatic aortic stenosis carries a poor prognosis.2 Medically treated patients with symptomatic AS have 1-year and 5-year survival rates of 60% and 32%, respectively.3 Aortic valve replacement (AVR) is the only effective treatment that alleviates symptoms and improves survival in patients symptomatic AS. In the ideal candidate, surgical AVR has an estimated operative mortality of 4%.4 However, the rates of operative mortality and postoperative complications increase with age and become significantly higher when surgery is done urgently and when pre-existent comorbidities such as coronary artery disease, poor left ventricular function, renal insufficiency, pulmonary disease, and diabetes are present.5,6 These factors are considered some of the major reasons for one third of patients with valve disease not being referred for surgery.7 Trans-catheter aortic valve implantation (TAVI) has opened the possibility for treating patients who, until now, had been left untreated as it was believed that their operative mortality outweighed the benefits offered by traditional AVR.
Before the onset of BAV in 1986, AVR was the only recommended therapy for patients with symptomatic severe AS or was the expectant observation if patients were thought to be “too old” or “high risk.”8,9 The concept of “old age” has continued to be redefined and has resulted in a moving target for comparison as these techniques have been evolving and the population has been aging. Currently, age is no longer considered a surgical contraindication, and very old patients (octogenarians and nonagenarians) are offered the option if they do not have significant physical or psychological comorbidities.10–12 The percentage of patients 90 years of age and over undergoing heart surgery has doubled from 1994 to 2001.11 Patients with poor left ventricular function are also more aggressively managed surgically.13–15 In Europe and the United States, a large number of patients with severe AS are still not offered valve replacement.7,16 In the 1990s, the early enthusiasm for BAV as a possible alternative to surgical aortic valve replacement in adult patients disappeared following the recognition of the problem of re-stenosis. The procedure appeared to provide only temporary benefit in symptoms and, at best, a modest survival benefit with a relatively high complication rate.17,18 As more experience was reported, there were discrepancies in reported results and complication rates. Whereas there is agreement with regard to the benefit of the procedure in neonates, infants, and young patients, the role of BAV in adults remains controversial as reflected in the updated American College of Cardiology (ACC) guidelines.19 For the high-risk older adult with severe calcific AS, the response from the cardiologist has been to limit the recommendation for BAV and for the cardiac surgeon to continue to broaden the inclusion of patients for surgery, regardless of age or left ventricular function. A large number of patients with severe AS and comorbidities that render them inoperable or very high risk still remain untreated, particularly when the risk assessment by the Parsonnet score or the EuroSCORE appears unfavorable.17 In spite of some patients being determined to be too sick for surgery, BAV is not offered to them in most centers because of its perceived limitations.7 TAVI was introduced in a subset of patients with severe AS who were not surgical candidates, and over the last 8 years, the technique has been refined dramatically.21 The results of early registry data show significant hemodynamic improvement without re-stenosis and have allowed its commercialization in Europe, but randomized control trials are still ongoing in the United States. On the basis of current results, TAVI carries the potential of offering a therapeutic option to patients who until recently have been left untreated and also revolutionizes the treatment of severe AS and prosthetic dysfunction. BAV plays an integral role in TAVI, and as a result, there has been increased interest in interventional cardiologists and surgeons in learning this technique.
The objectives of this chapter are to:
 Balloon Aortic Valvuloplasty
Balloon Aortic Valvuloplasty
Since the first reported cases, we have published their continuing experience, which now exceeds 1000 cases.22–28 Like others, immediate improvement in symptoms, hemodynamics, and left ventricular function could be obtained but the mid-term and long-term results were disappointing.17,18 In our hands, BAV remains a valuable palliative procedure for frail patients who are extremely old, often with compromised clinical status from concomitant coronary artery disease (CAD) and other extracardiac comorbidities. Most of the referred patients have been turned down by surgeons, and BAV is attempted as a “bridge to surgery” in about one third of the cases. The technique used now allows us to obtain improved hemodynamic results and reduced complications in this high-risk subset of patients.28
The goal of the procedure is to achieve a 100% increase in the aortic valve area. This requires attention to obtaining the maximum pressure exerted on the valve leaflets during balloon inflation, proper balloon sizing, and optimal contact with the valve structures. Improper techniques may explain the disparity of the results in the literature. The final valve area is a determinant of the prognosis, and in some reported series, the increase in the aortic valve area after the procedure was very modest.17,29,30 The results of BAV are limited by the pathology involved in the disease. Degenerative AS is its most common etiology and appears to be associated to a chronic inflammatory process.31–34 Unlike rheumatic mitral stenosis, commissural fusion is not the predominant feature in the majority of older patients with calcific AS, and as a result, commissural splitting is not the major mechanism of action in balloon dilatation of adult calcific AS.35 The primary mechanism of the balloon action in AS is fracture of nodular calcium deposits, thereby improving leaflet mobility, which allows increased valve opening and blood flow during left ventricular contraction.36 At full inflation, the rigid balloon can stretch the elastic component of the valve structures and the annulus. These elastic properties can result in immediate or early recoil, which are associated with an unsuccessful or suboptimal procedure result. Overstretching can result in tearing or rupture of the elastic and fibrocalcific components of the leaflets, the annulus, or the adjacent myocardium, leading to aortic insufficiency.37 Despite improvement in technique and materials, re-stenosis continues to plague BAV.18,38–40 Early re-stenosis occurring within hours or days is caused by early recoil and could be related to the pathology of the valve components, or inappropriate balloon diameter (because of size or insufficient inflation). The causes of re-stenosis occurring after several months may be multi-factorial, including the original degenerative process and an altered healing process with fibrosis and ossification.41,42 When patients develop recurrent symptoms, BAV can be repeated, usually after an interval of 12 to 24 months, and the dilations can be done serially.26–28,43,44 In some cases, the patient may be “bridged” to AVR or TAVI.19 Despite its limitations, BAV can provide symptomatic relief and modest survival benefit in selected patients who are very old or have comorbidities and who currently may have no other options.17–1929 BAV can be done using either the retrograde approach or the antegrade approach. The retrograde approach was first described by Lababidi in infants and children, and then by our group in adults.8,45 The antegrade approach was reported by Block, and comparisons between the techniques have been reported.46–48 There is no significant benefit to the antegrade approach compared with the retrograde approach except in patients with peripheral vascular disease or small vessel size.49 For both approaches, we typically perform the baseline hemodynamic study to confirm the presence of severe AS at the same setting as the planned BAV intervention.
The Retrograde Approach
Technique for Retrograde Crossing of the Native Aortic Valve
When using the appropriate technique, the stenotic aortic valve can be crossed within a few minutes in most cases. An Amplatz left coronary catheter 2 (AL-2) is commonly used for this task. A straight-tip, fixed-core, 0.035-inch guidewire is positioned at the tip of the catheter. In the 40-degree LAO projection, the catheter tip is positioned at the rim of the valve. The catheter is slowly pulled back, while a firm clockwise rotation is maintained to direct the catheter tip toward the center of the valve plane (Fig. 50-1, A). The guidewire is carefully moved in and out of the catheter tip, sequentially mapping the valve surface and exploring for the valve orifice. Once the wire crosses the valve, the catheter is advanced over the wire and positioned in the middle of the left ventricle (LV). The trans-valvular gradient is obtained using the side arm of the femoral sheath to record the aortic pressure. A dual-lumen multi-purpose or pigtail catheter may be used to reduce the peripheral augmentation of the systemic pressure. Cardiac output is then measured. The aortic valve area is calculated using Gorlin’s formula.50
Equipment Required for Balloon Aortic Valvuloplasty
Guidewire
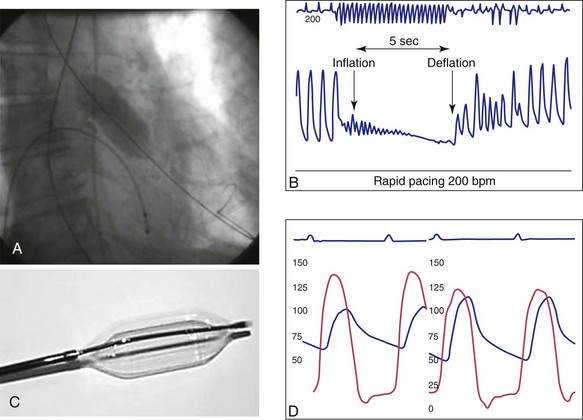
An extra-stiff Amplatz 0.035-inch, 270-cm length guidewire (Cook, Bjaeverskov, Denmark) is used to perform all catheter exchanges and to assist in stabilizing the valvuloplasty balloon during inflation, deflation, and withdrawal. Before inserting the wire, a large pigtail-shaped curve is formed at the distal end of the wire with a dull instrument (Fig. 50-2, A) to prevent ventricular perforation and to decrease ectopy.
Sheaths
The 8F arterial sheath is replaced over the extra-stiff wire with a 10F, 12F, or 14F sheath (Cook, Bjaeverskov, Denmark), depending on the balloon catheter required. The evolution of the technique has seen a reduction in the profile of the devices, reducing local complications at the femoral artery puncture site, which was previously the most common complication reported.51 Until recently, we have been using 12F to 14F sheaths, facilitating hemostasis by “preclosing” with a 10F Prostar device (Prostar, Abbott Vascular, Redwood City, Ca). Currently, since a 10F sheath is generally required, we are able to close the arteriotomy site using an 8F Angioseal device (Angioseal Vascular Closure Device, St. Jude Medical, Belgium) at the end of the procedure.
Rapid Ventricular Pacing
A 6F temporary bipolar pacing lead is positioned in the right ventricular posterior wall and connected to a pulse generator capable of pacing at up to 220 beats per minute (beats/min). Pacing and sensing parameters are determined and then the blood pressure response to pacing at 200 to 220 beats/min is evaluated. The rapid ventricular pacing (RVP) causes a precipitous fall of blood pressure to at least 50 mm Hg to be effective (Fig. 50-3). If this is not achieved at a rate of 200 beats/min, then the response is checked again at 220 beats/min. If 2 : 1 conduction block is seen, then the rate will need to be reduced to 180 beats/min or the lead position modified. The pacer is set on demand mode at 80 beats/min, serving as a backup in the event of a vagal episode or an interruption of AV conduction resulting in bradycardia or asystole in response to balloon inflations. The diagnostic catheter is removed from the LV over the extra-stiff wire, while the looped flexible segment of wire is carefully maintained in the LV cavity (see Fig. 50-1, B). The 8F sheath is replaced by the 10F sheath over the extra-stiff wire (see Fig. 50-2, B). A short extension tubing with a three-way stopcock attached is connected to a hand-held 30-mL luer-lock syringe filled with diluted contrast. The contrast is diluted at 15% contrast to 85% saline to reduce viscosity and thus facilitate the inflation–deflation cycles. After flushing the distal lumen and applying negative pressure on the balloon port, the balloon catheter is mounted on the extra-stiff wire and advanced into the aorta and allowed to rest above the aortic valve. At this time, the balloon is partially inflated and then completely deflated one or more times to completely purge it of air bubbles. De-airing the balloon in the ascending aorta has the advantage of maintaining the lowest balloon profile while crossing the aortic arch, thus decreasing the risk of atheromatous plaque dislodgement and embolization. The balloon catheter is advanced across the aortic valve, centering the valve between the two markers. In the past, before using RVP, it was always challenging to maintain the balloon in the optimal position during balloon inflation. The inflated balloon would tend to “pop” into the LV abruptly, striking the apex, or would “eject” itself back into the aorta with the possibility of disrupting atheromatous plaque, which could embolize.
Balloon Inflation
RVP and simultaneous forward pressure on the balloon catheter and forward pressure on the extra-stiff wire help stabilize the balloon during inflation. Traction on the guidewire causes forward movement on the balloon. Pushing the guidewire displaces the balloon in an aortic direction allowing for better positioning. There must be clear communication between the operators manipulating the balloon catheter and the pacing device. RVP is turned on, and balloon inflation is started quickly and with enough pressure to rapidly inflate the balloon (see Fig. 50-1, C) as soon as the blood pressure falls. RVP is continued for a few seconds after the balloon reaches maximal inflation. The balloon is rapidly deflated, the pacer is turned off, and the balloon is withdrawn from the valve. This step requires the coordination of the two operators to quickly allow restoration of antegrade flow while maintaining safe wire position in the LV. Rapid balloon deflation and restoration of blood flow are important to minimize the time of hypotension and hypoperfusion. We allow time for heart rate and blood pressure to return to preinflation parameters before proceeding to inflate the balloon again. Since the pressure gradient cannot be measured through the current generation of balloon catheters, it is important to assess the effects of the balloon dilation and the hemodynamic consequences by observing the wave form of the aortic pressure tracing as well as the heart rate response and rhythm and blood pressure recovery. A sudden change in waveform with loss of the dicrotic notch or falling diastolic blood pressure could indicate the presence of severe aortic regurgitation. An improvement in the pressure slope is suggestive of a successful procedure. If the balloon does not appear to be fully expanded, or there is no hemodynamic improvement, then repeat inflations are usually carried out before measuring the trans-aortic gradient again. The balloon catheter is removed while applying negative pressure on the balloon port, maintaining guidewire position in the LV. Particular care must be taken as the deflated balloon is drawn through the sheath. If resistance is encountered, it may be necessary to remove the catheter and the sheath together as a single unit. The residual gradient is then obtained by simultaneous measurement of pressures in the LV and in the aorta (Fig. 50-4). If there is a significant gradient, the next larger size balloon may be chosen, and the sequence is repeated. A pullback gradient is also obtained after the final balloon inflation. For the final results, the pacemaker is removed, the cardiac output is measured, and the final aortic valve area is calculated. An optimal result is considered to be doubling of the valve area or decreasing the gradient by 50% compared with the baseline value. Supra-aortic angiography to determine the presence and the severity of aortic regurgitation may be performed (see Fig. 50-1, D). If contrast cannot be used, assessment of the presence of aortic insufficiency and its severity may be performed by trans-thoracic echocardiography (TTE).
The Antegrade Trans-septal Approach
Results Using Contemporary Balloon Aortic Valvuloplasty Techniques
The results of our series of 141 consecutive patients with severe AS who underwent BAV (with the exception of patients undergoing percutaneous heart valve implantation) between January 2002 and April 2005 have been published.28 In this group of patients, the average age was 80.3 ± 10 years, 45% were women, and they were high risk for surgery or were inoperable. Eighty percent were in New York Heart Association (NYHA) functional class IV, with 28% of patients having poor left ventricular function (ejection fraction [EF] <30%). The procedure was done emergently for patients in cardiogenic shock in 5.6% of cases. BAV was done using the retrograde approach in 95% of the cases. The largest balloon was 23 mm in 84% of the procedures. The immediate results showed an increase in aortic valve area from 0.59 ± 0.19 to 1.02 ± 0.34 cm2 (P < 0.001) and a decrease in trans-valvular gradient from 49.3 ± 21.2 to 22.2 ± 11.8 mm Hg (P < 0.001). Post-BAV aortic regurgitation was grade 2 in 14%, grade 3 in 3.5%, and grade 4 in 1.4% of the cases. Six patients (4%) died. Nonfatal severe complications occurred in 9 patients (6%): 2 transient strokes, 5 episodes of complete atrioventricular (AV) block, and 2 severe aortic regurgitation. There were 8 vascular complications that did not require surgical repair. Discharge from the hospital was at 5.6 ± 3 days. In our series, the frequency of clinically apparent neurologic events was less than 2%. This compares favorably with the reported incidence of cerebrovascular events in a series of retrograde catheterizations of the aortic valve without intervention.52 We give heparin before crossing and then use a technique that minimizes trauma to the aortic valve structure during the attempted crossing. Although feared as a potentially fatal and disabling complication, embolization of atheromatous debris, which could break loose during the balloon’s impact on the valve, is, in fact, quite rare. Since many of these patients have concomitant cerebrovascular disease, hypotension and hypoperfusion during balloon inflation can also result in a neurologic event. Minimizing the duration of rapid ventricular pacing and balloon inflation is an important technical issue, and maintaining optimal heart rate and blood pressure during the procedure is crucial. Preventing, recognizing, and treating vagal reactions expeditiously are also important to avoid the possible neurologic consequences of hypotension. Improvements in procedures such as RVP and in vascular closure devices as well as continued experience have resulted in decreased complications despite an increasingly older and sicker population of patients. The large registries reported higher complication rates, possibly related to multiple participating centers, many of whose operators were reporting their first experience.18,51 The reduction of complications in our series, compared with the results of an early registry, is notable as shown in Table 50-1.
TABLE 50-1 Comparisons of Complication Rates in the Rouen Series and in the Mansfield Registry
| Complications | Mansfield Scientific Aortic Valvuloplasty Registry 1986–1988 (N = 492) | Rouen Series 2002–2005 (N = 141) |
|---|---|---|
| Procedural death | 2 (4.9%) | 3 (2.1%) |
| Postprocedural death (<7 days) | 12 (2.6%) | 3 (2.1%) |
| Cerebral embolic events | 11 (2.2%) | 2 (1.4%) |
| Transient ischemic attacks | 5 (1.1%) | 0 (0%) |
| Ventricular perforation with tamponade | 11 (2.2%) | 0 (0%) |
| Severe aortic insufficiency | 5 (1.1%) | 2 (1.4%) |
| Vascular complications (surgical repair) | 27 (5.5%) | 0 (0%) |
| Nonfatal arrhythmias | 5 (1.1%) | 5 (3.5%) |
| Other: myocardial infarction, sepsis, renal failure | 8 (1.6%) | 1 (1%) |
Current Perspectives of Balloon Aortic Valvuloplasty
The recently updated ACC/AHA guidelines for the management of patients with valvular heart disease continue to regard the role of BAV as controversial.19 There are no class I or IIa recommendations for BAV. The class IIb indications for adult patients with severe AS are (1) for patients who are at high risk for AVR because they are hemodynamically unstable and would be candidates for “a bridge to surgery” or (2) as a palliative procedure for patients with a serious comorbid condition which would preclude AVR. Because of improvements in the surgical technique, all older patients who are suitable candidates should receive surgical AVR.10,11,53,54 However, in the frail older patient with comorbidities, the risk for perioperative complications, individual patient preferences, and ethical and economical factors should be taken into consideration when considering AVR or a less invasive option. Other potential indications for BAV are:
At present, BAV remains a viable alternative for the management of selected patients with severe AS. BAV continues to play an important role in the management of AS, particularly as a palliative modality for the increasing population of older adults, in whom the risk of surgery is too high or not appropriate.27,28,44,56,57 Interventional cardiologists and surgeons should become familiar with this technique, particularly if they are interested in the trans-femoral or trans-apical TAVI, as it plays a crucial role in patient selection and valve implantation.
 Trans-Catheter Aortic Valve Implantation
Trans-Catheter Aortic Valve Implantation
Percutaneous catheter-based systems for the treatment of patients with valvular disease has been an exciting area for research since the mid-1960s. The initial animal investigations were performed by Davies in 1965, followed by Moulopoulos in 1971, Phillips in 1976, and Matsubara in 1992.58–61 These investigators reported on various catheter-based systems for temporary relief of aortic insufficiency, but no further human application was possible because of unsolved major limitations. A new era of investigations started with the development of endovascular stents, giving rise to the concept of balloon expandable valvular prosthesis. In 1992, Andersen et al reported their work in a porcine model, in which they evaluated a trans-luminal stented heart valve.62 Here again, despite encouraging experimental results, there was no development of human application. Subsequently, in 2000, Bonhoeffer and coworkers using a valve from a bovine jugular vein mounted within an expandable stent reported the feasibility of delivering such a device inside the native pulmonary valve of lambs; thereafter, they were able to perform the first successful human percutaneous replacement of a pulmonary valve in an RV–PA (right ventricle–to–pulmonary artery) prosthetic conduit with valve dysfunction.63,64 Our team in Rouen has been working since the early 1990s on the development of a catheter-based treatment for nonsurgical patients with severe calcific AS that could overcome the high re-stenosis rate seen after BAV. Early cadaver work in 1994 provided information on the ability to deploy a Palmaz stent in the aortic position and contributed to appropriate stent dimensions. In 1999, under the auspices of PVT (Percutaneous Valve Technologies, Fort Lee, NJ), an original catheter valve was developed and tested in the sheep model.65 In vitro testing confirmed the valve’s hemodynamic profile and durability. An original animal model of chronic aortic regurgitation, which allowed long-term evaluation of the catheter valve in the systemic circulation, was developed for in vivo testing.66 The first TAVI in a human was performed by our group in April 2002,21 and this was followed by an initial series of human implantations for compassionate use.67–69 Following the acquisition of PVT by Edwards LifeSciences in 2003, further modifications of the device (Cribier–Edwards and the Edwards-SAPIEN Heart Valve) preceded multi-center clinical trials. Results from other centers were published, confirming the feasibility of TAVI.70,71 The first series of patients with severe AS treated with the self-expanding CoreValve Revalving system was reported by Grube et al subsequently.72 Eight years after the first valve implantation, and after multiple device modifications, TAVI has been made commercially available in Europe, and the first multicenter randomized trial has been completed in the United States. To date, more than 50 TAVI procedures occur weekly, and the number continues to grow. TAVI is the most exciting advancement in the field of interventional cardiology, as it has provided innovative therapy and created a strong interaction among cardiac surgeons. This section will provide a review of patient selection, procedural techniques, results, and future strategies with balloon-expandable Edwards-SAPIEN valves and the Medtronic CoreValve. In addition, other valve prostheses in different stages of development will be mentioned (see Fig. 50-1).
Risk Stratification
Currently, TAVI is being offered to patients who are at high risk for surgical complications because of their age or comorbidities. Patients are considered to have a high operative risk when their scores are in the upper decile for mortality or have a 30-day mortality greater than 15%. Surgical risk is most commonly estimated by the Society of Thoracic Surgery Predicted Risk of Mortality (STS-PROM) and the European System for Cardiac Operative Risk Evaluation (EuroSCORE). The EuroSCORE has been validated in patients undergoing valvular surgery.73 Since the prevalence of high-risk patients was low in the initial population that generated this tool, the logistic EuroSCORE was then developed to more accurately predict the mortality rate in this patient population.74 However, this algorithm has been shown to persistently overestimate the mortality rate.75,76 The STS-PROM score is derived from the Society of Thoracic Surgeons (STS) database, a voluntary registry of practice outcomes, which estimates the risks of mortality, morbidity, renal failure, and length of stay after valvular and nonvalvular cardiac surgeries.77 This score has been shown to underestimate the true mortality rate after cardiac surgery, but it more closely reflects the operative and 30-day mortality for the highest-risk patients undergoing aortic valve replacement.78 The STS-PROM and the EuroSCORE provide an objective way to quantify risk. Although thorough, these risk scores do not include certain characteristics that would complicate surgery and increase operative mortality, such as: previous mediastinal irradiation, the presence of severe calcification in the thoracic aorta (porcelain aorta), anatomic abnormality of the chest wall, history of mediastinitis, liver cirrhosis, or the patient’s frailty. In addition, the algorithms were calculated from patients who underwent surgery, thus limiting their applicability to patients who were not considered surgical candidates. Clinical judgment and the patient’s level of independent functioning are subjective parameters that influence outcomes after cardiac surgery but are difficult to measure. The information from the PARTNER (Placement of AoRTic traNscathetER valves) trial will likely aid in the development of risk calculators that will more precisely estimate the risk for patients selected for TAVI.
Patient Screening
All patients considered for TAVI need to undergo:
Vascular Screening
After the screening process has been completed and the patients have been deemed eligible for TAVI, then the route of implantation needs to be determined. The CoreValve Revalving system may be delivered by the femoral or subclavian approach.79,80 With the Edwards-SAPIEN valve, two implantation routes are available: trans-apical and trans-femoral. Both delivery methods are comparable in success and complication rates. Selection depends on the tortuosity, calcification, and internal diameter of the femoral, external iliac, and common iliac arteries. The presence of abdominal aortic aneurysms or history of their repair would favor the use of the trans-apical approach or the subclavian approach. Vascular complications have been associated with significant mortality and may be prevented with appropriate screening.81 Safety should not be sacrificed if both approaches are available and patients are considered good candidates. Contrast angiography provides an appropriate screening tool for route selection.82 However, it does not provide a detailed determination of the vascular anatomy. By inserting a guidewire across the iliac arteries, the degree that the vessels will straighten can be evaluated; if the arteries persist with significant tortuosities after insertion of a stiff guidewire, a TA or subclavian approach is preferred. CTA allows for precise determination of the degree, extent, and localization of vascular calcification. In addition, three-dimensional vessel reconstruction and cross-section imaging allow for the precise determination of vessel luminal diameter. The minimal luminal diameter and the length of the segment with the minimal luminal diameter are the main considerations for selecting the delivery approach. Contrasted CT scans are preferred; however, if chronic renal insufficiency precludes the use of a fully contrasted study, then intra-arterial administration of a small contrast bolus or a noncontrasted CT may provide appropriate images for the necessary measurements.83 The use of intravascular ultrasound (IVU) is an invasive way of measuring the arterial diameter; however, it may provide unreliable dimensions because of image obliquity. The Edwards-SAPIEN valve requires minimal luminal diameters of 7 mm and 8 mm to allow for the 22F and 24F sheaths, respectively, which are required for valve delivery. The new Edwards-SAPIEN XT valve allows for the vessels of 6 mm and 6.5 mm, requiring an 18F and a 19F sheath, respectively. The CoreValve Revalving System allows for a vessel diameter of 6 mm for the insertion of an 18F sheath. Special attention needs to be paid at the level of the aorto-iliac and internal–external iliac artery bifurcations, as these areas tend to be involved in vascular complications (Table 50-2).
TABLE 50-2 Minimal Vessel Diameter Requirements for Arterial Trans-catheter Aortic Valve Implantation
| Valve Size | Sheath Size | Minimal Arterial Diameter |
|---|---|---|
| Edwards-SAPIEN | ||
| 23 mm | 22 French (F) | 7 mm |
| 26 mm | 24F | 8 mm |
| Edwards-SAPIEN XT | ||
| 23 mm | 18F | 6 mm |
| 26 mm | 19F | 6.5 mm |
| CoreValve | 18F | 6 mm |
The Edwards-SAPIEN Valve
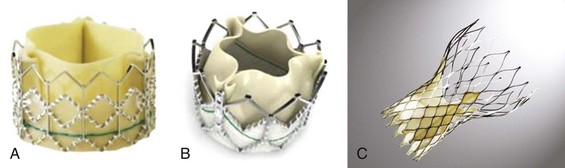
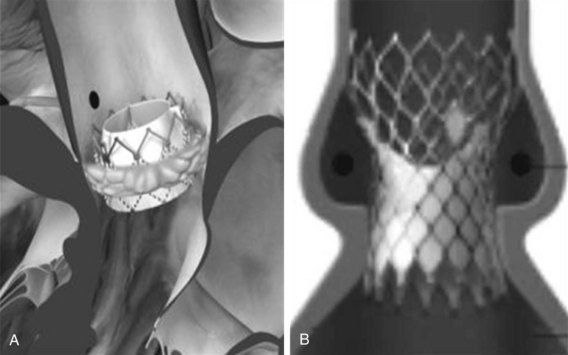
The Edwards-SAPIEN valve (Fig. 50-5) is a tri-leaflet bioprosthesis made of bovine pericardium mounted on a balloon-expandable stainless steel stent. It has been pre-treated to decrease calcification and functional deterioration. The stent has a fabric cuff on the ventricular side, which covers one half of the frame, limiting stent expansion and decreasing perivalvular insufficiency. Because of the lack of a sewing ring, the valve is oversized to the aortic annulus to ensure post-deployment stability and is currently available in two sizes: a 23-mm valve, with a stent height of 14.5 mm; and a 26-mm valve, with a stent height of 16 mm. In bench-top testing its durability is greater than 10 years. The Edwards-SAPIEN valve provides a larger effective orifice area and a lower hemodynamic profile compared with corresponding surgically implanted valves but has a higher incidence of perivalvular insufficiency.84 The new-generation device, Edwards-SAPIEN XT, currently commercially available in Europe, is made of a cobalt–chromium alloy, which provides the same radial strength while reducing the valve profile. This valve is currently approved for the trans-femoral approach and is under investigation for the trans-apical approach. In the future, 21-mm and 29-mm valves will be available.
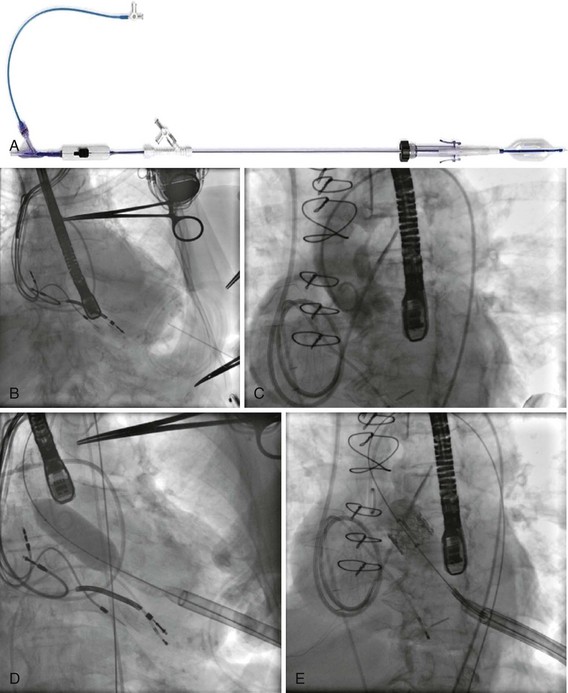
The Balloon Catheter
The valve is mounted on a custom-made balloon that is 30 mm in length, with balloon diameters corresponding to the sizes of prostheses, and ends in a nose cone that facilitates crossing the native valve (see Fig. 50-2). Its inflation profile decreases movement during inflation.
The Crimping Tool
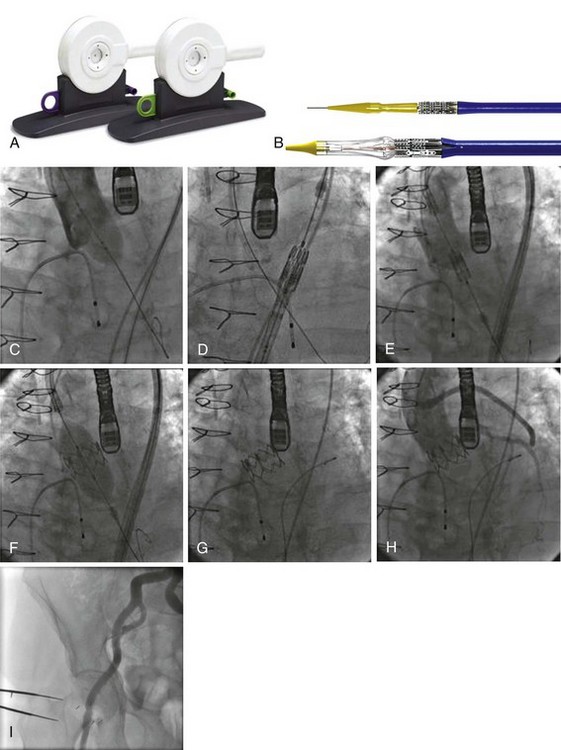
An original crimping tool (see Fig. 50-2) is used to manually and symmetrically compress the overall diameter of the percutaneous heart valve (PHV) from its expanded size to its minimal delivery profile. A cylindrical gauge is used to confirm the collapsed profile of the delivery system to ensure that it will move smoothly through the introducer sheath. A measuring ring is used to calibrate the balloon inflation to its desired size and to determine the amount of saline–contrast mixture in the syringe necessary for the proper inflation at the time of deployment.
The RetroFlex Guiding Catheter and the Ascendra Delivery System
The Retroflex catheter (Edwards LifeSciences Inc., Irvine, CA), an innovation to facilitate the PHV passage across the aortic arch from the retrograde approach, was initially evaluated by Webb (see Fig. 50-2).85 This catheter has a deflectable tip that changes direction when activated by the rotation of an actuator incorporated in the handle. The catheter is then used to direct the valve delivery system through the arterial system, around the aortic arch, and across the aortic valve, providing a less traumatic passage. The Retroflex catheter assists in centering and supporting the valve as it crosses the calcified and stenotic native valve. This system also provides precise positioning at the aortic annulus. The Novoflex catheter (Edwards LifeScience Inc. Irvine, CA), is a newer-generation catheter that allows loading the Edwards-SAPIEN XT prosthesis onto the balloon while in the body, decreasing the sheath size dramatically.
The Ascendra Delivery System (Edwards LifeSciences Inc., Irvine, CA) is the delivery catheter used for the trans-apical route (see Fig. 50-3). This catheter allows for easy valve manipulation to improve the orientation of the prosthesis.