TOTAL ANOMALOUS PULMONARY VENOUS CONNECTION
Introduction
In the previous chapters, transposition of the great arteries (TGA), tetralogy of Fallot (TOF) and tricuspid atresia were discussed. In this chapter, total anomalous pulmonary venous connection (TAPVC) will be reviewed. There are other pulmonary venous anomalies such as atresia of the common pulmonary vein, cor triatriatum, and stenosis/atresia of the individual pulmonary veins that may have similar embryologic origin, clinical features, and even therapeutic implications, but these will not be reviewed in this chapter.
In TAPVC, all the pulmonary veins drain into systemic venous structures. Most commonly, they are connected to a common pulmonary vein, which drains into the left innominate vein, superior vena cava (SVC), coronary sinus (CS), portal vein, or other rare sites. The pulmonary veins may also drain into multiple sites, or each of the pulmonary vein may be connected directly to the right atrium (RA).
TAPVC is the fifth most common cyanotic congenital heart disease (CHD) and occurs in 0.6 to 1.2 per 10,000 live births. The majority (68%) are diagnosed in the neonatal period.1 TAPVC is an isolated lesion (with the exception of patent foramen ovale [PFO]) in roughly two-thirds of babies and occurs along with other CHD and heterotaxia syndromes in the remaining one-third of infants. In some studies, female preponderance is reported,1 while in other studies, male preponderance,2 particularly of the infradiaphragmatic type, was observed. If not repaired, 50% of the infants are likely to survive up to 3 months and 20% up to 1 year of age, largely depending upon the type of TAPVC and the degree of pulmonary venous obstruction.2
Classification
Classifications of TAPVC are largely based on the site to which the connecting anomalous vein drains.3,4 Craig’s classification3 divided them into: type I, supracardiac connection draining into left innominate vein or right SVC; type II, cardiac connection draining into CS or RA; type III, infracardiac connection draining into the portal venous system; and type IV, mixed connections draining into multiple locations. Smith’s classification4 is much simpler, dividing them into supradiaphragmatic without pulmonary venous obstruction and infradiaphragmatic with obstruction. Although the supradiaphragmatic TAPVC is generally nonobstructive, obstructions were also seen in these, as reviewed elsewhere.5 However, the infradiaphragmatic TAPVC is almost always obstructive.
Supracardiac TAPVC is the most common at 45%, followed by cardiac at 25%, infracardiac at 25%, and mixed at 5%.6–10 Drainage into the left innominate vein is the most common type of TAPVC,11–13 while infradiaphragmatic type is most common form in the neonate.11–13
Pathophysiology
The entire pulmonary venous blood eventually returns into the RA in all types of TAPVC. Thus, mixing of systemic and pulmonary venous returns occurs in the RA. Consequently, right-to-left shunting across PFO or atrial septal defect (ASD) is a must for providing systemic flow. Most usually the fetal PFO14 persists and a restrictive atrial communication is not common during the neonatal period. The relative flow distribution to systemic (via PFO) and pulmonary (via tricuspid valve [TV]) circulations is dependent on relative compliances of the right ventricles (RVs) and left ventricles (LVs); these compliances are in turn determined by pulmonary and systemic vascular resistances, respectively.
In neonates with nonobstructive TAPVC, the pulmonary blood flow increases progressively as the pulmonary vascular resistance decreases with age. This will result in pulmonary over-circulation, which in turn leads to congestive heart failure. Enlargement of the RA and RV and dilatation of main and branch pulmonary arteries (PAs) will follow. In untreated patients, the increased pulmonary blood flow will produce pulmonary arteriolar medial hypertrophy and proliferation of intima, which in turn results in pulmonary hypertension and ultimately pulmonary vascular obstructive disease.
In infants with obstructive TAPVC, the pulmonary venous pressure is high, which produces reflex pulmonary arteriolar constriction with resultant high PA pressure and resistance, with consequent decrease in pulmonary blood flow. When the osmotic pressure of the blood surpasses the hydrostatic pressure in the capillaries, pulmonary edema ensues. Partial compensation may occur by increased pulmonary lymphatic drainage, development of substitute pulmonary venous bypass channels, and altered capillary permeability.
Pulmonary venous obstruction is present in all most all cases of infradiaphragmatic TAPVC. The obstruction is secondary to multiple causes such as high resistance to the passage of the blood via the hepatic sinusoids, constriction of the ductus venosus, intrinsic stenosis or extrinsic compression as the connecting vein passes through the diaphragm, or a combination thereof.5 The long connecting vein by itself offers impedance to the pulmonary venous return.
While obstruction is generally not present in the supradiaphragmatic TAPVC, pulmonary venous obstruction can and does occur in these types.5 Such obstruction may be located at several sites, may be intrinsic within the connecting vein itself or extrinsic by compression (of the vertical vein) between the left bronchus and left pulmonary artery (LPA) and is of varying degrees of severity. The intrinsic narrowing of the anomalous connecting vein may be at its junction with the common pulmonary vein, at its entry into the left innominate vein, SVC, azygos vein or RA, somewhere within the vein along its course itself, or a combination thereof.5 In addition, the left innominate vein, SVC or azygos vein into which the connecting vein enters may themselves be stenosed.5 However, obstruction is least likely when the pulmonary veins drain into the CS. Finally, the PFO itself may be obstructed.
Clinical Features
Clinical presentation is mainly dependent upon the extent of pulmonary venous obstruction.
The majority (~75%) of babies with obstruction will present within the first few days of life, and the remaining at a later time. The symptoms appear shortly after the first 12 hours of life; this is in contrast to respiratory distress syndrome, which usually becomes obvious at birth. These infants are acutely ill and have marked tachypnea, dyspnea, hypoxemia and metabolic acidosis. These signs and symptoms are as a result of severe pulmonary venous congestion. Physical examination is significant for rales and rhonchi in both lung fields. Cardiovascular findings include widely split second heart sound with an accentuated pulmonary component and no significant cardiac murmurs. At times, a nonspecific ejection systolic murmur along the left sternal border may be auscultated. The liver is usually enlarged. Pulmonary venous obstruction is seen in almost all infradiaphragmatic types and in approximately 50% of supradiaphragmatic types.
In babies without pulmonary venous obstruction, symptoms appear within the first month of life in more than half of the patients, and the remaining infants present during the first year of life. Some babies may be accidentally detected because of echocardiograms performed for an unrelated reason. These infants generally present with signs of congestive heart failure. Tachypnea, tachycardia, feeding difficulties, and failure to thrive are common symptoms at presentation. Physical examination reveals findings similar to those in infants with a secundum ASD in that there is a hyperdynamic right ventricular impulse, widely split and fixed second heart sound, an ejection systolic murmur of grade 2 to 3/6 intensity at the left upper sternal border, and a grade 1 to 2/6 intensity mid-diastolic flow rumble at the left lower sternal border. The pulmonary component of the second heart sound may be accentuated. An ejection systolic click and third and/or fourth heart sounds (multiple cardiac sounds) may be heard in some babies. Because of markedly increased pulmonary blood flow, cyanosis is minimal and may not be detected clinically. Pulse oxymetry may show O2 saturations in high 80s. Signs indicative of heart failure are usually present. A venous hum may be heard at the left or right upper sternal borders or in the infraclavicular regions in TAPVC of supracardiac type; the venous hum does not change by alterations in the position of the baby.
Noninvasive Evaluation
Chest X-Ray
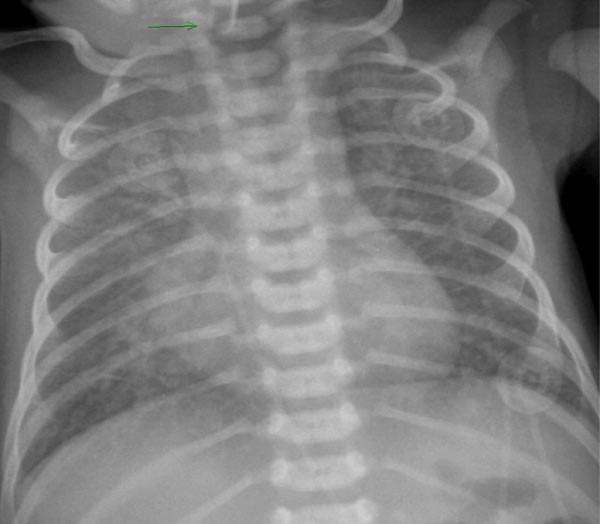
Roentgenographic findings are also dependent upon the degree of pulmonary venous obstruction. In babies with obstruction, the heart size is normal or mildly enlarged with evidence of distinct pulmonary edema with stippled densities and reticular pattern in the pulmonary parenchyma and unclear cardiac borders (Figure 31.1). Sometime, the reticular pattern may erroneously be interpreted as respiratory distress syndrome or group B streptococcal infection.
Figure 31.1. Chest x-ray of a neonate with TAPVC of infradiaphragmatic type demonstrating severe pulmonary venous congestion. Mild cardiac enlargement is also seen. Reproduced with permission from Neonatology Today
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree