History
General principles of the cardiovascular history
The suspicion of a cardiovascular abnormality may be raised initially by specific symptoms, but more commonly the presenting feature is the discovery of a cardiac murmur. Many children with a cardiac abnormality are asymptomatic because the malformation does not result in major hemodynamic alterations. Even with a significant cardiac problem, the child may be asymptomatic because the myocardium is capable of responding normally to the stresses placed upon it by the altered hemodynamics. A comparable lesion in an adult might produce symptoms because of coexistent coronary arterial disease or myocardial fibrosis.
In obtaining the history of a child suspected of cardiac disease, the physician seeks three types of data: those suggesting a diagnosis, assessment of severity, and indicating the etiology of the condition.
Diagnostic clues
Diagnostic clues and other more general factors include the following.
Gender
Certain cardiac malformations have a definite gender predominance. Atrial septal defect (ASD) and patent ductus arteriosus (PDA) are two to three times more likely in female than in male children. Coarctation of the aorta, aortic stenosis, and transposition of the great arteries occur more commonly in male children.
Age
The age at which a cardiac murmur or a symptom develops may give a diagnostic clue. The murmurs of congenital aortic stenosis and pulmonary stenosis are often heard on the first examination after birth. Ventricular septal defect (VSD) is usually first recognized because of symptoms and murmur at 2 weeks of age. The murmur of an ASD may not be discovered until the preschool examination. A functional (innocent) murmur is found in half of school-age children.
Severity of the cardiac condition
A physician should seek information that suggests the condition’s severity (e.g. dyspnea or fatigue).
Etiology
A physician should seek information that suggests an etiology of cardiac condition (e.g. maternal lupus).
Chief complaint and/or presenting sign
Certain presenting complaints and signs are more common in particular cardiac disorders and the “index of suspicion” aids the physician in organizing the data to make a differential diagnosis. For many of the signs and symptoms discussed later, noncardiac causes are often more likely than cardiac causes (e.g. acute dyspnea in a previously healthy 4-month-old infant with no murmur is more likely a result of bronchiolitis than of congestive heart failure). Therefore, a complete history must be integrated with the physical examination and other diagnostic studies to arrive at the correct cardiac diagnosis.
The most common symptoms or signs found in an outpatient setting are murmur, chest pain, palpitations, and near-syncope (fainting).
Murmur
Murmur is the most common presenting finding because virtually all children and adults with a normal heart have an innocent (normal) murmur sometime during their lifetime. Certain features are associated with an innocent murmur; the child is asymptomatic and murmurs appearing after infancy tend to be innocent. The murmur of atrial septal defect is one important exception.
Chest pain
Chest pain is a common and benign symptom in older children and adolescents, estimated to occur at some time in 70% of school-aged children. About 1 in 200 visits to a pediatric emergency room is for chest pain.
Chest pain rarely occurs with cardiovascular disease during childhood. Myocardial ischemic syndromes (e.g. Kawasaki disease with coronary artery aneurysms; hypertrophic cardiomyopathy) may lead to true angina. Patients with connective tissue disorders (e.g. Marfan syndrome) may have chest (or back) pain from aortic dissection. Although pericarditis may cause chest pain, it is almost always associated with fever and other signs of inflammation. Occasionally, chest pain accompanies supraventricular tachycardia. Most children with congenital cardiac malformations, including those who are fully recovered from surgery, do not have chest pain, and most children and adolescents who present with chest pain as their chief complaint do not have a cardiac malformation or disease.
Most chest pain is benign. It is usually transient, appearing abruptly, lasting from 30 seconds to 5 minutes and localized to the parasternal area. It is distinguished from angina by the absence of diaphoresis, nausea, emesis, and paresthesias in an ulnar distribution. Benign chest pain is “sharp,” not “crushing” like angina. It may also occur as a result of chest wall tenderness. Benign chest pain is typically well localized, sharp in character, short in duration (seconds to minutes), often aggravated by certain positions or movements, and occasionally can be induced by palpation over the area. These characteristics are strong evidence against cardiac cause for the pain. Some noncardiac conditions (e.g. asthma) may be associated with childhood chest pain. Benign pain is often described as “functional” because an organic cause cannot be found.
Palpitations
Palpitations, the sensation of irregular heartbeats, “skipped beats,” or, more commonly, rapid beats, are also common in the school-aged child and adolescent. They frequently occur in patients with other symptoms, such as chest pain, but often not simultaneously with the other symptoms. Palpitations are often found to be associated with normal sinus rhythm when an electrocardiogram is monitored during the symptom. Palpitations are not usually present in patients with known premature beats. Palpitations of sudden onset (approximately the time span of a single beat) and sudden termination suggest tachyarrhythmia.
Near-syncope
Near-syncope is a complex of symptoms that include vertigo and weakness. It is often induced by a postural change (orthostatic), is found commonly in older children and adolescents, and is almost always benign. The history often reveals little fluid and caloric intake beforehand. True syncope, characterized by complete loss of consciousness and loss of skeletal muscle tone, rarely results from a cardiac abnormality. It is often autonomic (vasovagal) in origin. Benign syncope is usually very brief in duration, often lasting only seconds. Benign syncope may follow a period of physical activity by several minutes; however, syncope during exercise often indicates a serious cardiac problem, such as aortic stenosis, arrhythmia, or myocardial abnormality. Because some life-threatening conditions (e.g. long QT syndrome) may result in syncope after a patient has been startled or has experienced an emotionally stressful situation, similar to benign syncope, an electrocardiogram is advisable for any child with a history of syncope. The family history should be explored for sudden death, syncope, seizures, SIDS, swimming deaths, and single-occupant motor vehicle fatalities.
The symptoms of dyspnea and fatigue must be carefully explored since they can occur in a variety of conditions, including cardiovascular conditions. They need to be interpreted with regard to the patient’s age and psychologic factors.
Dyspnea
Dyspnea (labored breathing) is different from tachypnea (rapid breathing). It is a symptom present in patients with pulmonary congestion from either left-sided cardiac failure or other conditions that raise pulmonary venous pressure or from marked hypoxia. Dyspnea is manifested in neonates and infants by rapid, grunting respirations associated with retractions. Older children complain of shortness of breath. The most common causes in children are asthma and bronchitis, whereas in the first year of life it is often associated with pulmonary infections or atelectasis.
Fatigue
Fatigue on exercise must be distinguished from dyspnea as it has a different physiologic basis. In neonates and infants, fatigue on exercise is indicated by difficulty while feeding. The act of sucking while feeding requires energy and is “exercise.” It is manifest by infants by stopping frequently during nursing to rest and the feeding may take an hour or more.
- Cyanotic congenital heart disease (arterial oxygen desaturation).
- Congestive cardiac failure (inadequate myocardial function).
- Severe outflow obstructive conditions or those causing cardiac filling impairment (inadequate cardiac output).
Fatigue on exercise or exercise intolerance is a difficult symptom to interpret because other factors, such as motivation or amount of training, influence the amount of exercise that an individual can perform. To assess exercise intolerance, compare the child’s response to physical activity with that of peers and siblings or with their previous level of activity.
The remaining symptoms are found more commonly in neonates and infants.
Growth retardation
Growth retardation is common in many children who present with other cardiac symptoms within the first year of life.
Infants with cardiac failure or cyanosis
Infants with cardiac failure or cyanosis show retarded growth, which is more marked if both are present. Usually, the rate of weight increase is more delayed than that of height. The cause of growth retardation is unknown, but it is probably related to inadequate caloric intake due to dyspnea and fatigue during feeding and to the excessive energy requirements of congestive cardiac failure.
Growth
Growth may also be retarded in children with a cardiac anomaly associated with a syndrome, such as Down syndrome, which in itself causes growth retardation.
Developmental milestones
Developmental milestones requiring muscle strength may be delayed, but usually mental development is normal. To assess the significance of a child’s growth and development, obtaining growth and development information about siblings, parents, and grandparents is helpful.
Congestive cardiac failure
Congestive cardiac failure leads to the most frequently described symptom complex in infants and children with cardiac disease. In infants and children, 80% of instances of heart failure occur during the first year of life; these are usually associated with a cardiac malformation. The remaining 20% that occur during childhood are related more often to acquired conditions. Infants with cardiac failure are described as slow feeders who tire when feeding, this symptom indicating dyspnea on exertion (the act of sucking a bottle). The infant perspires excessively, presumably from increased catecholamine release. Rapid respiration, particularly when the infant is asleep, is an invaluable clue to cardiac failure in the absence of pulmonary disease. The ultimate diagnosis of cardiac failure rests on a compilation of information from the history, the physical examination, and laboratory studies such as chest X-ray and echocardiography. Management of congestive cardiac failure is discussed in Chapter 11.
Respiratory infections
Respiratory infections, particularly pneumonia and RSV, are frequently present in infants and, less commonly, in older children with cardiac anomalies, especially those associated with increased pulmonary blood flow (left-to-right shunt) or with a greatly enlarged heart. The factors leading to the increased incidence of pneumonia are largely unknown but may be related to compression of the major bronchi by either enlarged pulmonary arteries, an enlarged left atrium, or distended pulmonary lymphatics.
Atelectasis may also occur, particularly in the right upper or middle lobe, in children with greatly increased pulmonary blood flow, or in the left lower lobe in children with a cardiomyopathy and massively dilated left atrium and ventricle.
Cyanosis
Cyanosis is a bluish or purplish color of the skin caused by the presence of at least 5 g/dL of reduced hemoglobin in capillary beds. The desaturated blood imparts a bluish color to the appearance, particularly in areas with a rich capillary network, such as the lips or oral mucosa. The degree of cyanosis reflects the magnitude of unsaturated blood. Mild degrees of arterial desaturation may be present without cyanosis being noted. Usually, if the systemic arterial oxygen saturation is less than 88%, cyanosis can be recognized – this varies with skin pigmentation, adequacy of lighting, and experience of the observer. A minimal degree of cyanosis may appear as a mottled complexion, darkened lips, or plethoric fingertips. Clubbing develops with more significant degrees of cyanosis.
Cyanosis is classified as either peripheral or central.
Peripheral cyanosis
Peripheral cyanosis, also called acrocyanosis, is associated with normal cardiac and pulmonary function. Related to sluggish blood flow through capillaries, the continued oxygen extraction eventually leads to increased amounts of desaturated blood in the capillary beds. It typically involves the extremities and usually spares the trunk and mucous membranes. Exposure to cold is the most frequent cause of acrocyanosis, leading to blue hands and feet in neonates and circumoral cyanosis in older children. Peripheral cyanosis disappears upon warming. The normal polycythemia of neonates may contribute to the appearance of acrocyanosis.
Central cyanosis
Central cyanosis is related to any abnormality of the lungs, heart, or hemoglobin that interferes with oxygen transport from the atmosphere to systemic capillaries. Cyanosis of this type involves the trunk and mucous membranes in addition to the extremities. A variety of pulmonary conditions, such as atelectasis, pneumothorax, and respiratory distress syndrome, can cause cyanosis. Areas of the lungs, although not ventilated, are perfused, and blood flowing through that portion of the lung remains unoxygenated. Thus, desaturated blood returns to the left atrium and mixes with fully saturated blood from the ventilated portions of the lungs. Rarely, dysfunctional hemoglobin disorders, such as excessive levels of methemoglobin, result in cyanosis because hemoglobin is unable to bind normal quantities of oxygen.
Squatting
Squatting is a relatively specific symptom, occurring almost exclusively in patients with tetralogy of Fallot. It has virtually disappeared except in countries where children with tetralogy of Fallot do not have access to surgery. When experiencing a hypercyanotic or “tet” spell, cyanotic infants assume a knee/chest position, whereas older children squat in order to rest. In this position, the systemic arterial resistance rises, the right-to-left shunt decreases, and the patient becomes less desaturated.
Neurologic symptoms
Neurologic symptoms may occur in children with cardiac disease, particularly those with cyanosis, but are seldom the presenting symptoms. Brain abscess may accompany endocarditis in severely cyanotic children. Stroke may be seen in cyanotic patients and the rare acyanotic child with “paradoxical” embolus occurring via an atrial septal defect. Stroke may also occur intra- or postoperatively, or as a result of circulatory support devices, and in cardiomyopathy, and rarely in children with arrhythmia. In otherwise apparently normal children, seizures stem from arrhythmias, such as the ventricular tachycardia seen in the long QT syndrome, and may be the sole presenting symptom.
Prenatal history
A prenatal history may also suggest an etiology of the cardiac malformation if it yields information such as maternal rubella, drug ingestion, other teratogens, or a family history of cardiac malformation. In these instances, a fetal echocardiogram is often performed to identify possible anomalies of the heart or other organ systems.
Family history
The physician should obtain a complete family history and pedigree to disclose the presence of congenital cardiac malformations, syndromes, or other disorders, such as hypertrophic cardiomyopathy (associated with sudden death in young persons) or long QT syndrome (associated with a family history of seizures, syncope, and sudden death).
Other facts obtained on the history that may be diagnostically significant will be discussed in relation to specific cardiac anomalies.
Physical examination
When examining a child with suspected cardiac abnormalities, the physician may focus too quickly on the auscultatory findings, overlooking the general physical characteristics of the child. In some patients, these findings equal the diagnostic value of the cardiovascular findings.
Cardiac abnormalities are often an integral part of generalized diseases and syndromes: recognition of the syndrome can often provide a clinician with either an answer or a clue to the nature of the associated cardiac disease. These syndromes are discussed in Chapter 2.
Vital signs
Blood pressure
In all patients suspected of cardiac disease, examiners should record accurately the blood pressure in both arms and one leg. Doing this aids in diagnosis of conditions causing aortic obstruction, such as coarctation of the aorta, recognition of conditions with “aortic runoff,” such as patent ductus arteriosus, and identification of reduced cardiac output.
Many errors can be made in obtaining the blood pressure recording. The patient should be in a quiet, resting state, and the extremity in which blood pressure is being recorded should be at the same level as the heart. A properly sized blood pressure cuff must be used because an undersized cuff causes false elevation of the blood pressure reading. A slightly oversized cuff is unlikely to affect readings greatly. Therefore, blood pressure cuffs of various sizes should be available. A guide to the appropriate size for each age group is given in Table 1.1. Generally, the width of the inflatable bladder within the cuff should be at least 40% of the circumference of the limb, and the bladder length should encompass 80–100% of the circumference of the limb at the point of measurement. In infants, placing the cuff around the forearm and leg rather than around the arm and thigh is easier.
Table 1.1 Recommended Dimensions for Blood Pressure Cuff Bladders.
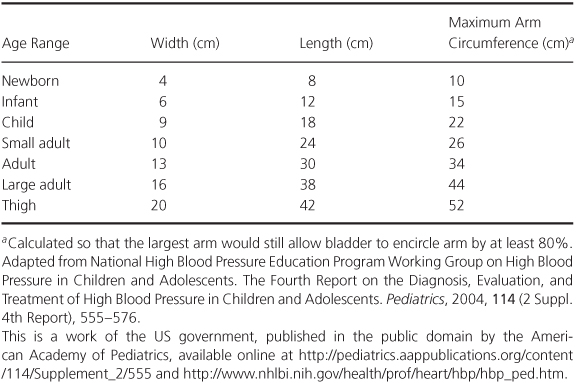
Although a 1-inch-wide cuff is available, it should never be used because it leads uniformly to a falsely elevated pressure reading except in the tiniest premature infants. A 2-inch-wide cuff can be used for almost all infants.
Failure to pause between readings does not allow adequate time for return of venous blood trapped during the inflation and may falsely elevate the next reading.
Methods
Four methods of obtaining blood pressure can be used in infants and children – three manual methods (flush, palpatory, and auscultatory) and an automated method (oscillometric).
For manual methods, the cuff should be applied snugly and the manometer pressure quickly elevated. The pressure should then be released at a rate of 1–3 mmHg/s and allowed to fall to zero. After a pause, the cuff can be reinflated. Pressure recordings should be repeated at least once.
Flush method
A blood pressure cuff is placed on an extremity, and the hand or foot is tightly squeezed. The cuff is rapidly inflated, and the infant’s hand or foot is released. As the cuff is slowly deflated, the value at which the blanched hand or foot flushes reflects the mean arterial pressure. By connecting two blood pressure cuffs to a single manometer and placing one cuff on the arm and the other cuff on the leg, simultaneous blood pressure can be obtained.
Palpation
Palpation can also be used in infants. During release of the pressure from the cuff, the pressure reading at which the pulse appears distal to the cuff indicates the systolic blood pressure. A more precise but similar method uses an ultrasonic Doppler probe to register the arterial pulse in lieu of palpating it.
Auscultation
In an older child, blood pressure can be obtained by the auscultatory method: in the arm, by listening over the brachial artery in the antecubital space, or in the leg and in the thigh, by listening over the popliteal artery. The pressure at which the first Korotkoff sound (K1) is heard represents the systolic pressure. As the cuff pressure is released, the pressure at which the sound muffles (K4) and the pressure at which the sound disappears (K5) should also be recorded. The diastolic blood pressure is located between these two values.
Automated
Automated methods have largely replaced the manual methods. They are widely used in ambulatory, hospital, and intensive care settings. These oscillometric methods uses a machine that automatically inflates and deflates the cuff while monitoring pulse-related air pressure fluctuations within the cuff. Deflation is performed in a stepwise fashion, and at each step the machine pauses for 2 seconds or less while the cuff pressure oscillations are recorded. The amplitude of these pulsatile oscillations begins to increase as the cuff pressure falls to the level of the systolic blood pressure, reaches a maximum amplitude at a cuff pressure equal to mean blood pressure, and diminishes as cuff pressure falls to diastolic levels. Because the method depends on measurement of faint pulsatile pressure oscillations, irregular heart rhythm (e.g. atrial fibrillation), conditions with beat-to-beat variability in pulse pressure (e.g. the pulsus alternans of heart failure or mechanical ventilator-induced changes), and patient movement may lead to inaccurate or absent readings.
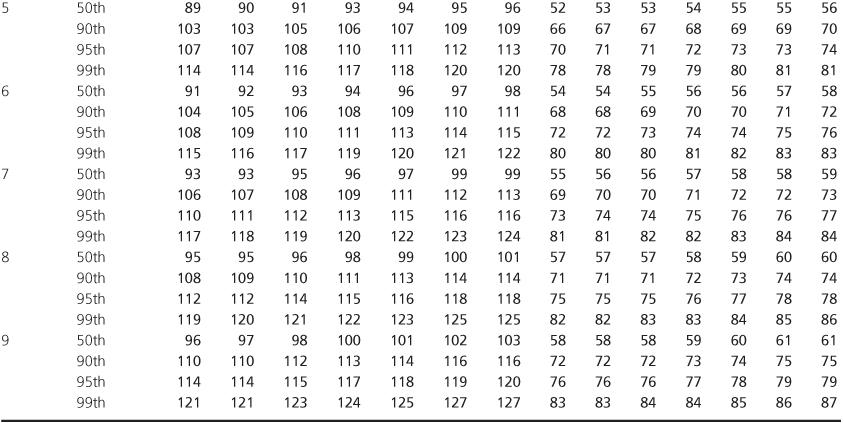
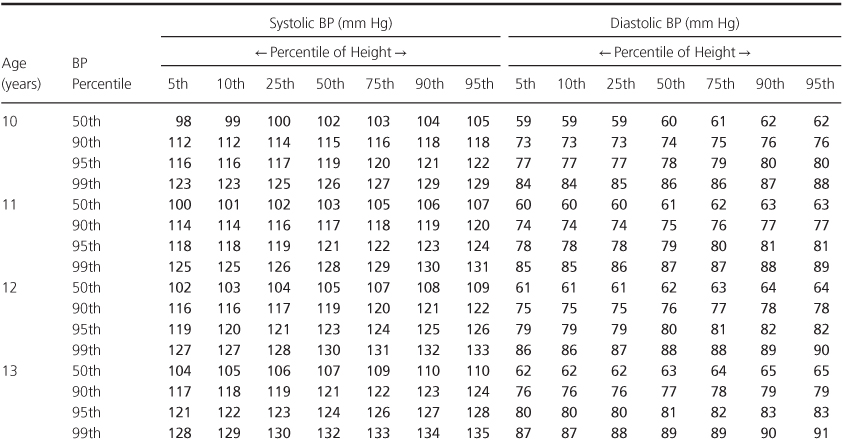
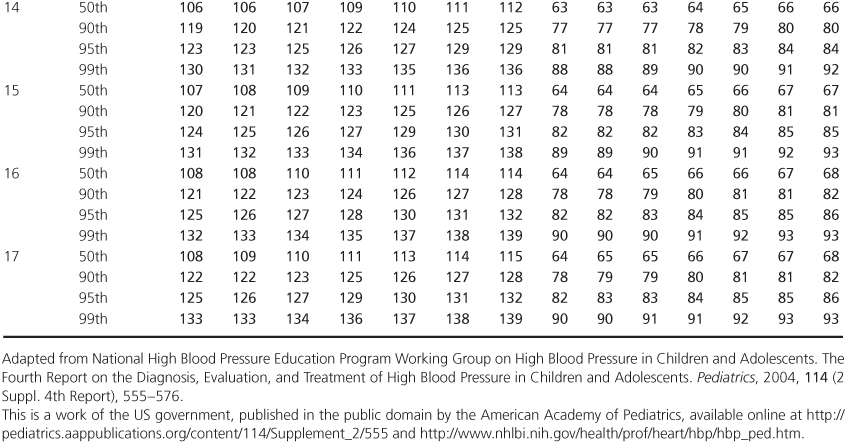
Normal values
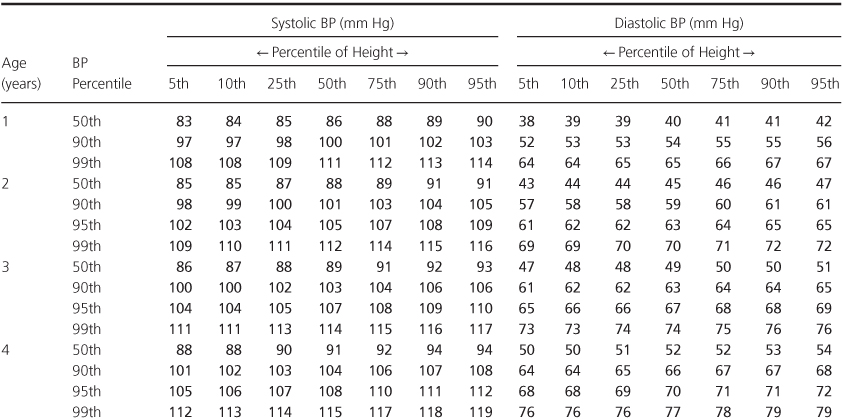
The normal blood pressure values for different age groups are given in Figure 1.1 and Tables 1.2 and 1.3. The blood pressure in the leg should be the same as that in the arm. Leg blood pressure should also be taken with an appropriate-sized cuff, usually larger than the cuff used for measurement of the arm blood pressure in the same patient. Since the same-sized cuff is frequently used at both sites, the pressure values obtained may be higher in the legs than in the arms. Coarctation of the aorta is suspected when the systolic pressure is 20 mmHg lower in the legs than in the arms.
Figure 1.1 Upper limits of blood pressure for (a) girls and (b) boys from birth to 1 year of age. From Report of the Second Task Force on Blood Pressure Control in Children. Pediatrics, 1987, 79, 1–25. The material is a work of the US Government in the public domain; it is reprinted with acknowledgement from the American Academy of Pediatrics.
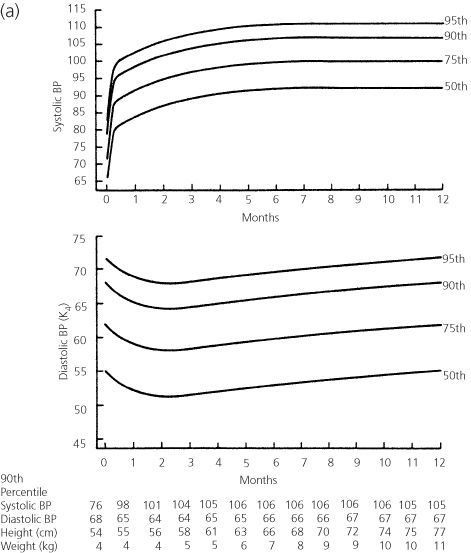
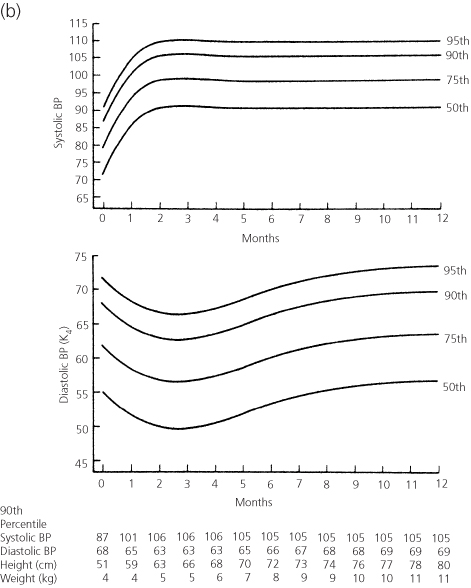
Table 1.2 Blood Pressure Levels for Boys by Age (1–17 years) and Height Percentile.
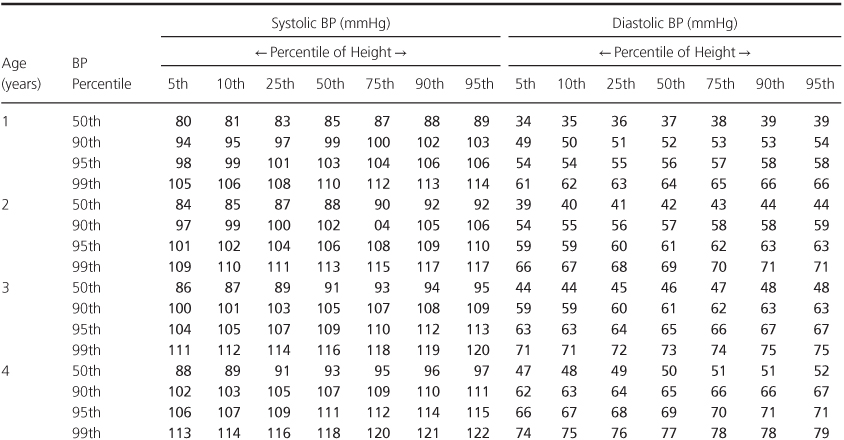
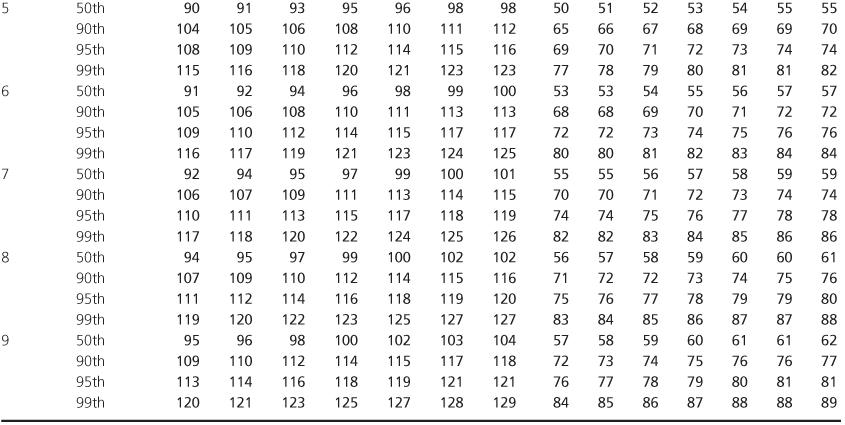
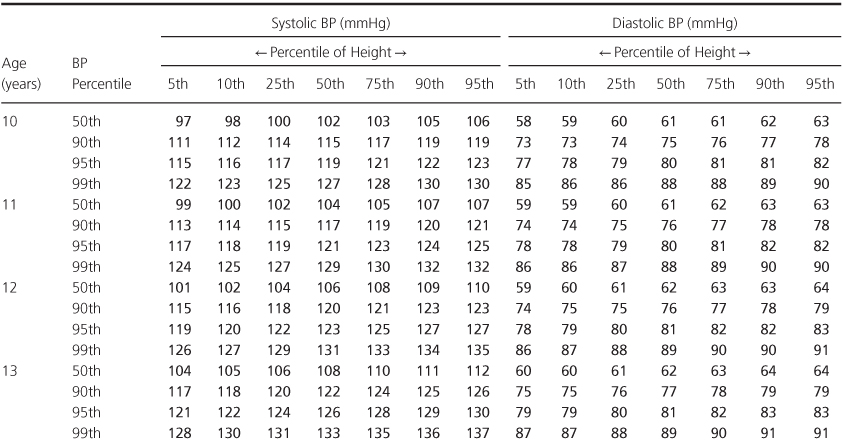
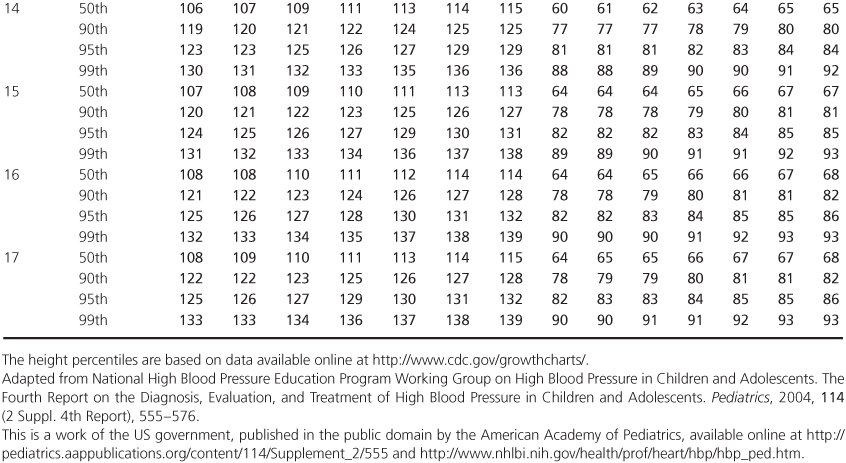
Table 1.3 Blood Pressure Levels for Girls by Age (1–17 years) and Height Percentile.
Blood pressure must be recorded properly by listing in the patient’s record the systolic and diastolic pressure values, the method of obtaining the pressure, the extremity used, and whether upper- and lower-extremity blood pressures were measured simultaneously or sequentially. When using automated methods requiring nonsimultaneous measurement, recording the heart rate measured with each pressure reading may be helpful, since wide rate variations may give a clue to varying states of anxiety and may help in the interpretation of differing pressure values.
Pulse pressure
Pulse pressure (the difference between the systolic and diastolic pressures) normally should be approximately one-third of the systolic pressure. A narrow pulse pressure is associated with a low cardiac output or severe aortic stenosis. Pulse pressure widens in conditions with an elevated cardiac output or with abnormal runoff of blood from the aorta during diastole. The former occurs in such conditions as anemia and anxiety, whereas the latter is found in patients with conditions such as PDA or aortic regurgitation.
Pulse
In palpating a child’s pulse, not only the rate and rhythm but also the quality of the pulse should be carefully noted, as the latter reflects pulse pressure. Brisk pulses reflect a widened pulse pressure, whereas weak pulses indicate reduced cardiac output and/or narrowed pulse pressure. Coarctation of the aorta, for example, can be considered by comparing the femoral with the upper-extremity arterial pulses. Mistakes have been made, however, in interpreting the quality of femoral arterial pulses. Palpation alone is not sufficient either to diagnose or to rule out coarctation of the aorta. Blood pressures must be taken in both arms and one leg.
Respiratory rate and effort
The respiratory rate and respiratory effort should be noted. Normal values for the respiratory rate are given in Table 1.4. Although the upper limit of normal respiratory rate for an infant is frequently given as 40 breaths per minute, observed rates can be as high as 60 breaths per minute in a normal infant; the respiratory effort in such infants is easy. Difficulty with breathing is indicated by intercostal or suprasternal retractions or by flaring of the alae nasae. Premature infants or neonates may show periodic breathing, so the rate should be counted for a full minute.
Table 1.4 Normal Respiratory Rates at Different Ages.
| Age | Rate (breaths/min)a |
| Birth | 30–60 (35) |
| First year | 30–60 (30) |
| Second year | 25–50 (25) |
| Adolescence | 15–30 (15) |
a Respiratory rates (breaths/min) vary with changes in mental state and physical activity. Sleeping rates are slower and are indicated in parentheses. Depth of respirations and effort expended by the patient are equally or more important than the rate itself.
Cardiac examination
Inspection
Cardiac examination begins with inspection of the thorax. A precordial bulge may be found along the left sternal border in children with cardiomegaly. The upper sternum may bulge in children with a large left-to-right shunt and pulmonary hypertension or with elevated pulmonary venous pressure.
Palpation
Several findings may be discovered by palpation; the most important is the location of the cardiac apex, an indicator of cardiac size. Obviously, if the apex is in the right hemithorax, there is dextrocardia.
Apical impulse
In infants and children under 4 years of age, the apex impulse, which is the most lateral place that the cardiac impulse can be palpated, should be located in the fourth intercostal space at the mid-clavicular line. In older children, it is located in the fifth intercostal space at the midclavicular line. Displacement laterally or inferiorly indicates cardiac enlargement.
Thrills
These are best identified by palpation of the precordium with the palmar surfaces of the metacarpophalangeal and proximal interphalangeal joints. Thrills are coarse, low-frequency vibrations occurring with a loud murmur, and are located in the same area as the maximum intensity of the murmur. In any patient suspected of congenital heart disease, the suprasternal notch also should be palpated but with a fingertip. A thrill at this site indicates a murmur originating from the base of the heart, most commonly aortic stenosis, less commonly pulmonary stenosis. In patients with PDA or aortic insufficiency, the suprasternal notch is very pulsatile.
Heaves
Forceful, outward movements of the precordium (heaves) indicate ventricular hypertrophy. Right ventricular heaves are located along the right sternal border, and left ventricular heaves are located at the cardiac apex.
Percussion
Percussion of the heart can substantiate estimation of cardiac size in addition to that obtained by inspection and palpation.
Auscultation of the heart
Auscultation of the heart provides perhaps the most useful diagnostic information and should be performed in a systematic way to obtain optimum information.
Instrumentation
A good stethoscope is a must. It should have short, thick tubing, snug-fitting earpieces, and both a bell and a diaphragm. Low-pitched sounds and murmurs are heard best with the bell, and high-pitched sounds with the diaphragm. For most children, a ¾-inch bell and a 1-inch diaphragm are suitable for auscultation, although an adult-sized bell and diaphragm are preferable if adequate contact can be made with the chest wall. A diaphragm 1 inch in diameter can be used in children of all ages, since only part of the diaphragm need be in contact with the chest wall to transmit sound. Smaller sized diaphragms provide poor sound transmission.
Position and technique
In infants, initially auscultate through the clothing despite the often-quoted admonition that auscultation should never be performed in such a manner. Sometimes removing the clothes disturbs the child and results in a fussy state that precludes adequate auscultation. After the initial period of listening, the clothing can be removed to listen further. Make certain that the chest pieces of the stethoscope are warm.
With children between the ages of 1 and 3 years, listening is easier if they are sitting on their parent’s lap because children of this age are often frightened by strangers. In older children, they can sit on the examination table and the examination can proceed as in adults.
When auscultating, sitting alongside the child is helpful. This position is neither fatiguing to the examiner nor threatening to the child.
Auscultation of the heart should proceed in an orderly, stepwise fashion. Both the anterior and posterior thorax are auscultated with the patient in the upright position. Then the precordium is re-examined with the patient reclining. Each of the five major areas (aorta, pulmonary, tricuspid, mitral, and back) is carefully explored. Both the bell and diaphragm should be used in auscultation of each site. High-pitched murmurs and the first and second heart sounds are heard better with the diaphragm; low-pitched murmurs and the third heart sound are most evident with the bell. The diaphragm should be applied with moderate pressure; the bell must be applied with only enough pressure for uniform contact and not enough force to stretch the underlying skin into a “diaphragm,” which alters the sensitivity to low frequencies. When auscultating the heart, attention is directed not only to cardiac murmurs but also to the quality and characteristics of the heart sounds.
Physiologic basis of auscultation
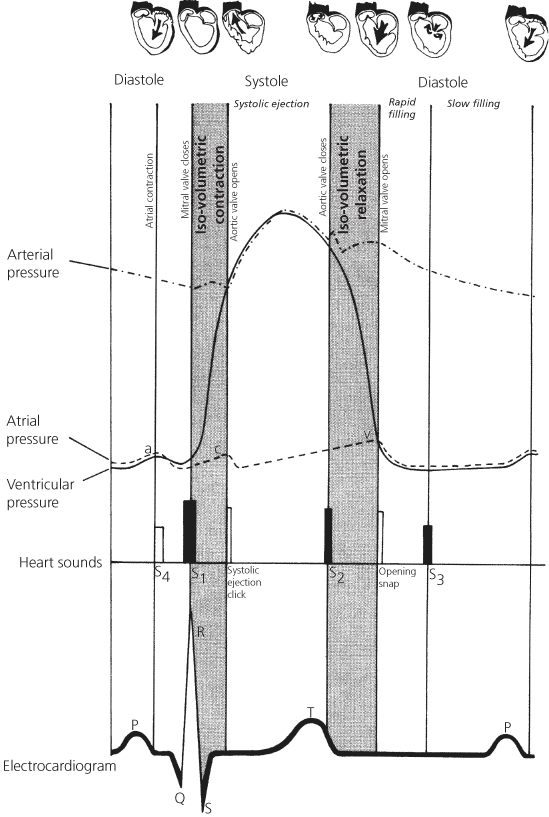
The events and phases of the cardiac cycle should be reviewed. Figure 1.2 represents a modification of a diagram by Wiggers and shows the relationship between cardiac pressures, heart sounds, and electrocardiogram. In studying this diagram, relate the events both vertically and horizontally.
Figure 1.2 Relationship between cardiac pressures, electrocardiogram, heart sounds, and phases of the cardiac cycle. S1, first heart sound; S2, second heart sound, etc.
Interpretation of cardiac sounds and murmurs
The timing and meaning of cardiac sounds and murmurs are easily understood by considering their location within the cardiac cycle and the corresponding cardiac events. Although the origin of heart sounds remains controversial, we will discuss them as originating from valvar events.
Heart sounds
The first heart sound (S1) represents closure of the mitral and tricuspid valves (Figure 1.2) and occurs as the ventricular pressure exceeds the atrial pressure at the onset of systole. In children, the individual mitral and tricuspid components are usually indistinguishable, so the first heart sound appears single. Occasionally, two components of this sound are heard. Splitting of the first heart sound can be a normal finding.
The first heart sound is soft if the impulse conduction from atrium to ventricle is prolonged. This delay allows the valves to drift closed after atrial contraction. The first heart may also be soft if myocardial disease is present.
The first heart sound is accentuated in conditions with increased blood flow across an AV valve (as in left-to-right shunt) or in high cardiac output.
The second heart sound (S2) is of great diagnostic significance, particularly in a child with a cardiac malformation. The normal second heart sound has two components which represent the asynchronous closure of the aortic and pulmonary valves. These sounds signal the completion of ventricular ejection. Aortic valve closure normally precedes closure of the pulmonary valve because right ventricular ejection is longer. The presence of the two components, aortic (A2) and pulmonic (P2), is called splitting of the second heart sound (Figure 1.3).
Figure 1.3 Respiratory variations in splitting of second heart sound. In a normal individual, P2 (pulmonary component of second heart sound) is delayed on inspiration. Wide splitting occurs in conditions prolonging right ventricular ejection. Paradoxical splitting occurs in conditions delaying A2 (aortic component of second heart sound). P2 changes normally with inspiration. Thus, the interval between P2 and A2 narrows on inspiration and widens on expiration.
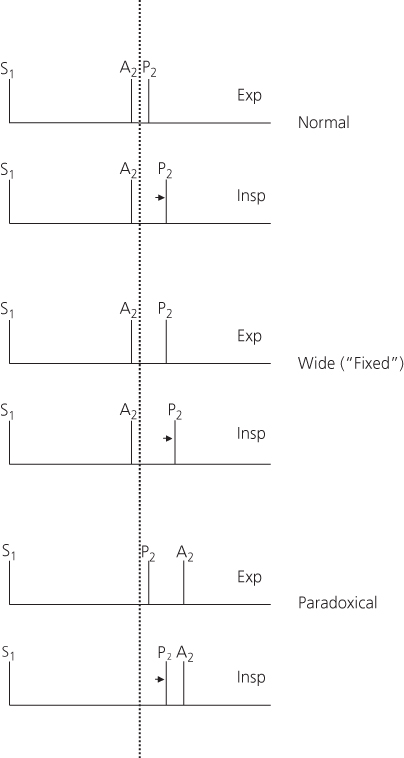
The time interval between the components varies with respiration. Normally, on inspiration the degree of splitting increases because a greater volume of blood returns to the right side of the heart. Since ejection of this augmented volume of blood requires a longer time, the second heart sound becomes more widely split on inspiration. On expiration, the degree of splitting is shortened.
- Conditions in which the right ventricle ejects an increased volume of blood (e.g. ASD – but not VSD).
- Obstruction to right ventricular outflow (e.g. pulmonary stenosis).
- Delayed depolarization of the right ventricle (e.g. complete right bundle branch block).
Thus, wide splitting and paradoxical splitting of the second heart sound occur from similar cardiac abnormalities but on opposite sides of the heart. Paradoxical splitting is associated with severe left-sided disorders.
Intensity of P2. In assessing a child with a cardiac anomaly, particular attention also should be directed towards the intensity of the pulmonic component (P2) of the second heart sound. The pulmonic component of the second sound is accentuated whenever the pulmonary arterial pressure is elevated, whether this elevation is related to pulmonary vascular disease or to increased pulmonary arterial blood flow. In general, as the level of pulmonary arterial pressure increases, the pulmonic component of the second heart sound becomes louder and closer to the aortic component.
Single second heart sound. The finding of a single second heart sound usually indicates that one of the semilunar valves is atretic or severely stenotic because the valve involved does not contribute its component to the second sound. The second heart sound also is single in patients with persistent truncus arteriosus (common arterial trunk) because there is only a single semilunar valve or whenever pulmonary arterial pressure is at systemic levels, and the aortic and pulmonary artery pressure curves are superimposed.
Third heart sound (S3) may be present in a child without a cardiac anomaly but may be accentuated in pathologic states. This sound occurs early in diastole and represents the transition from rapid to slow filling phases. In conditions with increased blood flow across either the mitral valve (as in mitral regurgitation) or the tricuspid valve (as in ASD), the third heart sound may be accentuated. A gallop rhythm found in congestive cardiac failure often represents exaggeration of the third heart sound in the presence of tachycardia.
Fourth heart sounds (S4) are abnormal. Located in the cardiac cycle late in diastole, they occur with the P wave of the electrocardiogram and exist synchronous to the atrial “a” wave. They are found in conditions in which either the atrium forcefully contracts against a ventricle with decreased compliance, as from fibrosis or marked hypertrophy, or when the flow from the atrium to the ventricle is greatly increased. The fourth heart sound may be audible as a presystolic gallop, particularly if tachycardia is present.
Systolic ejection clicks are abnormal and occur at the time the semilunar valves open. Therefore, they mark the transition from the isovolumetric contraction period to the onset of ventricular ejection. Ordinarily this event is not heard, but in specific cardiac conditions, a sound (systolic ejection click) may be present at this point in the cardiac cycle and because of its timing be confused with a split first heart sound.
Systolic ejection clicks indicate the presence of a dilated great vessel, most frequently from poststenotic dilation. These sharp, high-pitched sounds have a clicky quality. Ejection clicks of aortic origin are heard best at the cardiac apex or over the left lower thorax when the patient is in a supine position; they vary little with respiration. Aortic ejection clicks are common in patients with valvar aortic stenosis or a bicuspid aortic valve with concomitant poststenotic dilation. Ejection clicks may also originate from a dilated pulmonary artery, as present in pulmonary valvar stenosis or significant pulmonary arterial hypertension. Pulmonic ejection clicks are best heard in the pulmonary area when the patient is sitting and vary in intensity with respiration. Ejection clicks in patients with a stenotic semilunar valve occur more commonly in mild or moderate cases; they may be absent in patients with severe stenosis.
Clicks are not associated with subvalvar stenosis since there is no poststenotic dilation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


