11 Thrombolytic Intervention
Atherosclerotic plaque rupture with subsequent thrombus formation through an intricate series of interactions among the coronary artery endothelium, exposed subendothelium, circulating platelets, and coagulation factors leading to occlusion of an epicardial coronary artery are the pathophysiologic underpinnings of ST-elevation myocardial infarction (STEMI). The “open-artery” hypothesis, which postulates that early reperfusion of the occluded artery translates into myocardial salvage and ultimately improved survival, is the basis of reperfusion therapy. The evidence for the benefit of thrombolytic therapy as a reperfusion strategy in STEMI is indisputable. Pivotal placebo-controlled randomized trials of patients with STEMI from the 1980s, beginning with the GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico) and ISIS-2 (Second International Study of Infarct Survival) trials, proved the value of early thrombolysis by reducing mortality by approximately 30% (Table 11-1).1,2 These studies catalyzed the acceptance of thrombolysis as standard therapy for reperfusion in patients with acute MI and ushered in the thrombolytic era, a term that characterized the revolution in attitude among the medical community toward this disease. While primary PCI has superseded thrombolysis as the preferred reperfusion strategy in most instances, the recently updated American College of Cardiology/American Heart Association (ACC/AHA) STEMI guidelines continue to favor thrombolytic therapy over primary PCI as the primary reperfusion strategy in patients who meet the following conditions: (1) present early (<3 hours from symptom onset), (2) have no invasive option (catheterization laboratory [cath-lab] unavailable; vascular access issues), (3) face a significant delay in receiving PCI (prolonged transport time; door-to-balloon minus door-to-needle time of >60 minutes; or door-to-balloon time of >90 minutes), (4) are not in cardiogenic shock, and (5) have no contraindications to thrombolytic therapy (Table 11-2).1,3 However, because of concerns about bleeding complications, incomplete patency, and early reocclusion rates with thrombolytic therapy, combined with the fact that more primary PCIs are safely and successfully being performed at smaller hospitals without on-site cardiac surgery back-up, thrombolytic therapy has been surpassed by primary PCI as the treatment modality for STEMI associated with better clinical outcomes (Fig. 11-1).4–7 While PCI has evolved into the preferred reperfusion strategy, particularly at high-volume, experienced centers, approximately 50% of patients with acute MI present to hospitals that are not PCI-capable.8 Moreover, data from the National Registry of Myocardial Infarction (NRMI-4) suggest that door-to-balloon times remain poor with a median time of 180 minutes for patients transferred from one hospital to another for primary PCI, even within the framework of the current STEMI guidelines, indicating that there are still a substantial number of patients who experience an inordinate delay in mechanical reperfusion therapy in the “real world.”9 This was further evidenced by a cohort study of 68,439 patients presenting to PCI-capable hospitals with STEMI between 1999 and 2002, which suggested that most STEMI patients received reperfusion therapy (68.7% PCI and 54.2% fibrinolysis) during “off hours” (weekdays 5 P.M. to 7 A.M. and weekends) and that these patients experienced significant delays to PCI but not to thrombolytic therapy.10 Therefore, thrombolytic therapy remains an important tool in the therapeutic armamentarium for treating STEMI, albeit with significant limitations. This chapter reviews the historical basis of thrombolytic intervention, summarizes current thrombolytic agents and adjunctive therapies, and highlights the evidence for its current role in reperfusion therapy for STEMI, laying the groundwork for future progress in this field.
TABLE 11-1 Summary of Initial Randomized Clinical Trials for Thrombolytic Therapy in ST Elevation Myocardial Infarction
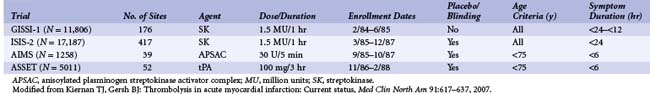
TABLE 11-2 Contraindications to Thrombolytic Therapy in Patients with ST Elevation Myocardial Infarction
| Absolute contraindications |
| Relative contraindications |
 Thrombolytic Agents
Thrombolytic Agents
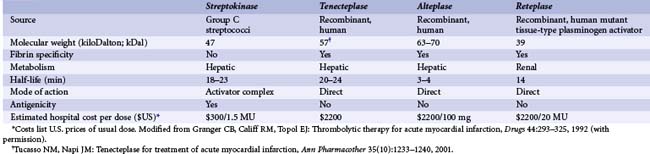
Thrombolytic therapy was born in 1933, when Tillett and Garner described the fibrinolytic activity of β-hemolytic streptococci leading to the first therapeutic attempt by Tillett and Sherry in 1948 to dissolve a fibrinous pleural effusion.11,12 Over 20 years later, in 1971, the results of the first randomized controlled trial demonstrating the benefit of thrombolytic therapy using streptokinase in acute MI were published.13 In 1981, Rentrop and colleagues showed that intracoronary streptokinase was effective in the lysis of coronary thrombi with the use of coronary angiography, and Markis et al., demonstrated myocardial salvage with the same therapy, thus providing proof of the validity of the concept.14,15 Shortly thereafter, human tissue plasminogen activator was isolated from a melanoma cell line16 and was demonstrated to be effective in reducing mortality in acute MI.17 Current thrombolytic therapy comprises a class of agents known as plasminogen activators, which directly or indirectly convert the proenzyme plasminogen into plasmin. Plasmin is a nonspecific serine protease that catalyzes the degradation of fibrin, fibrinogen, prothrombin, and factors V and VII, thus disrupting the coagulation cascade and thrombus generation.18 Plasminogen activators include the fibrin-specific agents such as alteplase (tissue-type plasminogen activator [t-PA]), single-chain urokinase plasminogen activator (scu-PA), tenecteplase (TNK) and staphylokinase (SAK), which enzymatically convert plasminogen into plasmin, as well as the non–fibrin-specific agents such as streptokinase, anistreplase (anisoylated plasminogen streptokinase activator complex [APSAC]) and urokinase. Reteplase (recombinant plasminogen activator [r-PA]) and lanoteplase (novel plasminogen activator [n-PA]) have intermediate fibrin specificity. Currently, four thrombolytic agents are approved for use by the U.S. Food and Drug Administration (FDA) and are used most commonly worldwide: streptokinase, alteplase, reteplase, and tenecteplase. The last three agents were developed via recombinant deoxyribonucleic acid (DNA) technology to improve fibrin-specificity and to increase the duration of activity to enable bolus dosing.18 The major thrombolytic agents are briefly reviewed with regard to mechanism of action and thrombolytic profile (Table 11-3). Two novel fibrin-specific agents, monteplase (MT-PA) and palmiteplase (YM866), are modified recombinant tissue plasminogen activators with relatively long half-lives, which have shown potential in preclinical and small-scale clinical studies but have not been tested in larger randomized trials. Amediplase [K(2) tu-PA] is a fibrin-specific hybrid plasminogen activator with improved clot penetration in animal models and is undergoing evaluation in Europe for the treatment of STEMI, but no major clinical studies with this agent have been published to date. A novel thrombin-activated plasminogen analogue (BB-10153), which would theoretically be activated only at the site of a developing thrombus, was studied in a small phase II dose-escalation clinical trial in 50 STEMI patients.19 This trial showed thrombolysis in MI (TIMI) 3 flow in 29% to 43% of patients over a range of bolus doses, with no major cardiovascular adverse events at 30 days of follow-up and relatively few bleeding events (3 TIMI major bleeds, 0 intracranial hemorrhage). The efficacy of this agent will need to be evaluated in larger phase III studies.
 Major Historical Comparative Thrombolytic Trials
Major Historical Comparative Thrombolytic Trials
The GISSI-2/International trial with 20,891 patients with STEMI within 6 hours of symptom onset randomly assigned to either alteplase or streptokinase demonstrated no difference in mortality between streptokinase and alteplase with or without subcutaneous heparin (intravenous heparin was rarely used in this trial) but showed a higher rate of ischemic stroke in the alteplase group.20 The ISIS-3 trial with 41,299 patients randomized to alteplase, streptokinase, or anistreplase demonstrated equivalence in mortality reduction among the three agents but found that streptokinase was associated with the lowest overall rates of stroke (1.1%) and intracerebral hemorrhage (0.3%).21 Intravenous heparin was not used in this trial; as it has been borne out collectively through other studies of the fibrin-specific plasminogen activators, adjunctive heparin—while not critical to achieve thrombolysis—is important to sustain infarct vessel patency through the avoidance of rethrombosis. The Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries I (GUSTO-I) trial with 41,021 patients promulgated the benefit of “accelerated” alteplase plus intravenous heparin in STEMI leading to a 15% relative and 1% absolute reduction in mortality (or 10 lives saved per 1000 patients treated).22 This study included an angiographic component, which led to the major finding that early and complete infarct vessel patency was tightly linked to a reduction in mortality.23 The Reteplase (r-PA) Angiographic Phase I International Dose-finding Study (RAPID-I) trial suggested a nonsignificant 30-day mortality benefit with reteplase compared with alteplase (1.9% vs. 3.9%), and the RAPID-II trial showed a significant improvement in coronary artery patency defined as TIMI-2 flow within 90 minutes favoring reteplase over alteplase (85.2% vs. 77.2%, P = 0.03).24,25 The GUSTO III trial with 15,021 patients failed to show true equivalence of reteplase to “accelerated” alteplase but demonstrated a trend toward equivalence, especially for death and disabling stroke. These findings, combined with the more convenient double-bolus administration, rendered reteplase a viable option for thrombolysis.26 The Assessment of the Safety and Efficacy of a New Thrombolytic 2 (ASSENT-2) trial of 16,949 patients randomized to receive either tenecteplase or alteplase found similar mortality rates, but the highly fibrin-specific tenecteplase was notable for significantly less bleeding compared with alteplase.27 While this result was tempered by a relatively high rate of intracerebral hemorrhage in both groups, lower rates of overall bleeding (26.1% vs. 28.4%, P < 0.0003) and blood transfusions (4.3% vs. 5.5%, P = 0.0002) were observed with tenecteplase compared with alteplase.
 Timing of Thrombolytic Therapy
Timing of Thrombolytic Therapy
Early Treatment
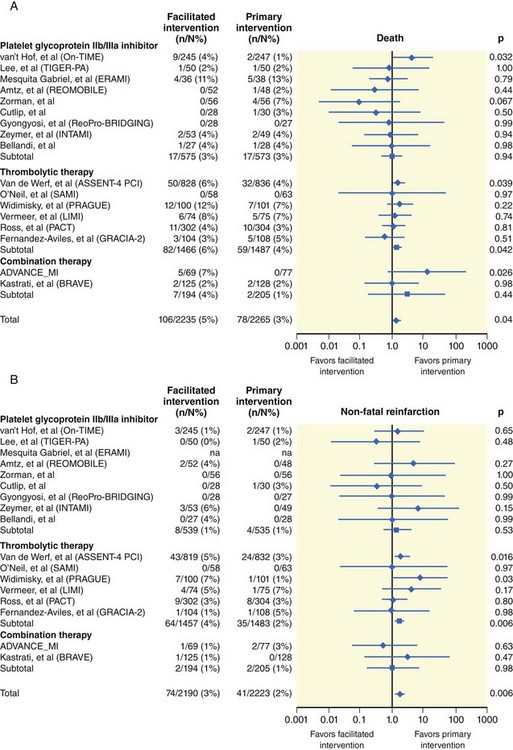
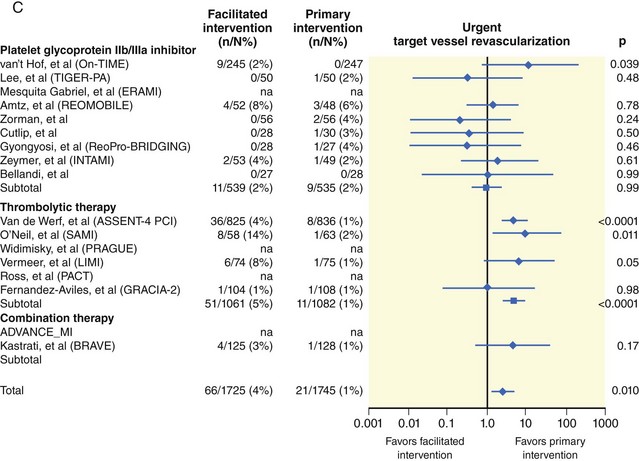
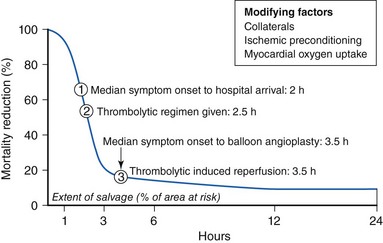
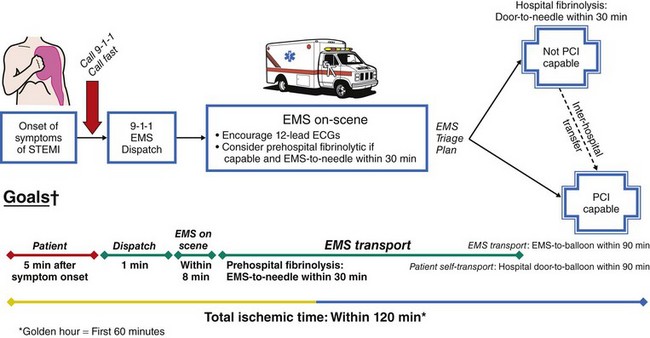
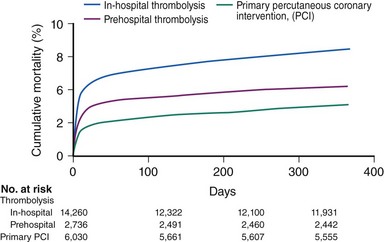
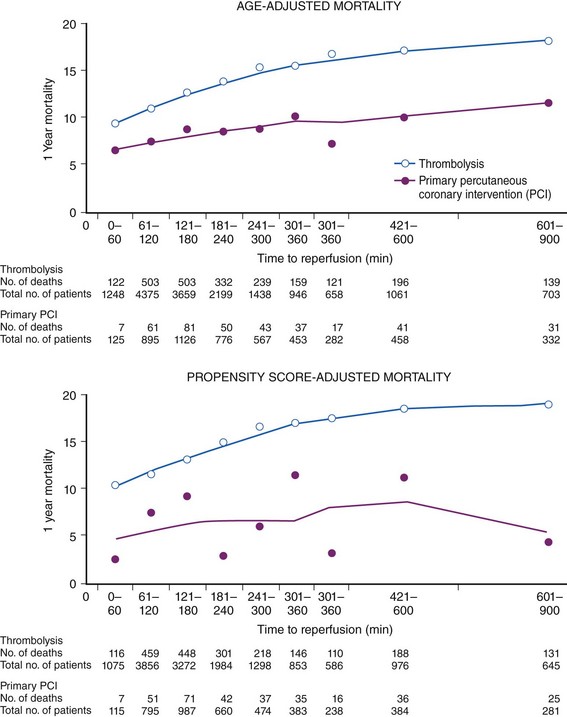
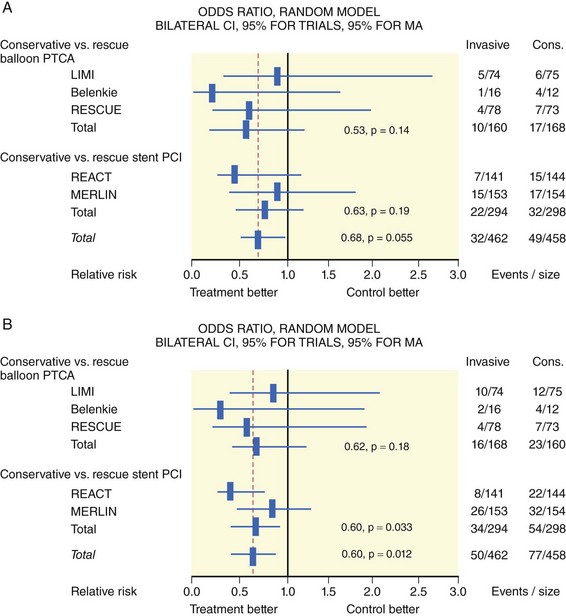
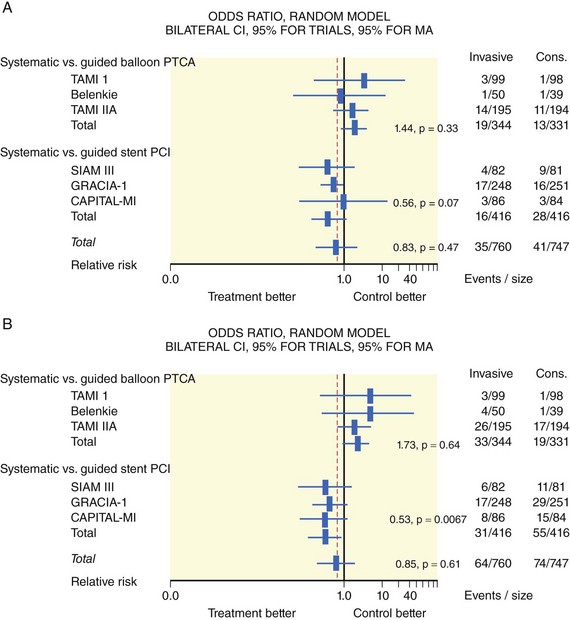
The degree of myocardial salvage following acute MI is clearly related to timely reperfusion, reinforcing the “time is myocardium” principle with the 2- to 3-hour time point representing the critical window to minimize morbidity and mortality from this disease process (Fig. 11-2).28 A meta-analysis of six randomized controlled trials of prehospital and in-hospital thrombolysis for acute MI including 6434 patients showed reduced time to thrombolysis (104 vs. 162 minutes, P = 0.007) and reduced all-cause hospital mortality (odds ratio [OR], 0.83; 95% confidence interval [CI] 0.70–0.98) with prehospital fibrinolysis.29 Subsequent randomized trials have reproduced these findings in populations in the United States as well as in Europe and have demonstrated the feasibility of safe and effective prehospital fibrinolysis administered by paramedics.30–32 Current AHA/ACC guidelines for STEMI management endorse the administration of pre-hospital fibrinolysis by appropriately trained personnel to patients with no contraindications to thrombolytic therapy (Fig. 11-3).3 Despite the evidence supporting the potential benefit of prehospital fibrinolysis, logistical challenges, including implementation of systems for rapid prehospital diagnosis and training of personnel in the appropriate administration of thrombolytic agents and adjunctive therapies, have limited the widespread acceptance and adoption of prehospital fibrinolysis.33,34 On the basis of the current ACC/AHA STEMI guidelines, in-hospital thrombolytic therapy should be administered to those patients who present within 3 hours of symptom onset with a large MI if a significant delay in primary PCI (the preferred reperfusion strategy) is anticipated and there is no evidence of cardiogenic shock.1 In patients who present after 3 hours, primary PCI appears to be superior to both prehospital and in-hospital thrombolysis in terms of myocardial salvage, infarct size, and mortality, possibly because of time-dependent thrombus resistance to thrombolytic agents35,36 (Fig. 11-4). There appears to be an early and sustained difference in favor of primary PCI versus thrombolysis as a function of time to reperfusion. A large registry study of 26,205 patients showed that it was not until approximately the 6- to 7-hour mark that age-adjusted 1-year mortality for primary PCI declined to the rates observed with thrombolysis administered within 2 hours (Fig. 11-5).36 Even if transfer to another facility is required, the benefit of PCI over thrombolysis with regard to the combined reduction in death, re-infarction, and disabling stroke at 30 days (driven primarily by re-infarction) is sustained in those patients with STEMI presenting less than 12 hours from symptom onset.7,37 However, the net benefit of primary PCI over thrombolysis may be neutralized if the door-to-balloon time for PCI is 60 minutes longer than door-to-needle time for thrombolysis, given that every 30-minute delay in the interval from symptom onset to balloon inflation is associated with a 7.5% increased risk of death at 1 year.38 In patients who present within 3 to 12 hours of symptom onset and when this delay is anticipated, thrombolysis is considered a viable alternative. Ting et al., have developed an evidence-guided approach for selecting the optimal reperfusion strategy based on incurred ischemia time (or transport time) and fixed ischemia time (or duration of symptoms) (Table 11-4).39 In 30% to 40% of patients who fail thrombolysis (<70% ST segment resolution at 90 minutes or ongoing chest pain), rescue PCI should be performed, given the benefit of this strategy (Fig. 11-6).40–42 There is also accumulating evidence supporting the benefit of performing routine cardiac catheterization and PCI within 24 hours or earlier in select cases regardless of the success of thrombolysis (Fig. 11-7).43–45 This “pharmaco-invasive” approach is discussed in more detail later in this chapter.
TABLE 11-4 Guide for Selecting a Reperfusion Strategy for Patients with ST Elevation Myocardial Infarction*
| Transport Time (Incurrent Ischemia Time) | Duration of Onset of Symptoms (Fixed Ischemia Time) | |
|---|---|---|
| <3 HR | >3 HR | |
| <30 min | Primary PCI and GP IIb/IIa | Primary PCI and GP IIb/IIa† |
| 30–60 min | Thrombolytic agent and clopidogrel‡ | Primary PCI and GP IIb/IIa† |
| >60 min | Thrombolytic agent and clopidogrel‡ | Thrombolytic agent and clopidogrel or Primary PCI and GP IIb/IIa‡ |
* Patients treated with thrombolytic agents should be immediately transferred to a PCI-capable facility in the event of failure to reperfuse. GP IIb/IIa, platelet glycoprotein IIb/IIa inhibitor; PCI, percutaneous coronary intervention.
† Based on the American Heart Association/American College of Cardiology guidelines.
Adapted with permission from Ting HH, Yang EH, Rihal CS: Narrative review: Reperfusion strategies for ST-segment elevation myocardial infarction, Ann Int Med 145:610–617, 2006.)
The “facilitated PCI” approach with early administration of a thrombolytic agent in anticipation of mechanical revascularization is also discussed later in this chapter as well as in Chapter 19, Acute MI Intervention.
Late Treatment
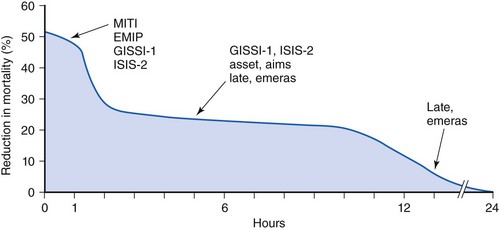
Large mega-trials of thrombolytic therapy have suggested that most of the benefit of this strategy is largely confined to the first 12 hours following symptom onset and that many of the complications such as serious bleeding and latent myocardial rupture occur with late thrombolysis. Two specific trials, the Late Assessment of Thrombolytic Efficacy (LATE) trial and the Estudios Multicentrico Estreptoquinasa Republica Americas Sud (EMERAS) trial addressed the issue of late thrombolysis and showed no demonstrable survival benefit beyond the 12-hour mark with alteplase or streptokinase, respectively, with excessive serious bleeding complications in the latter trial.46,47 The Fibrinolytic Therapy Trialists’ Collaboration pooled analysis of 52,892 patients enrolled into eight placebo-controlled trials (excluding LATE) showed significant benefit up to but not beyond 12 hours from symptom onset.48 The available data provide cogent evidence that there is a “golden” first hour and a “dim” 12th hour after symptom onset, illustrating the narrow therapeutic window for thrombolytic therapy (Fig. 11-8). Health care systems should develop and implement plans to minimize the delays in patient triage and facilitate timely initiation of reperfusion therapy.
 Adjunctive Therapies
Adjunctive Therapies
Aspirin
The standard adjuvant pharmacotherapy for all patients with acute MI undergoing thrombolysis should include 162 to 325 mg/day of aspirin in the absence of a documented allergy to aspirin.1,49 The benefit of aspirin therapy in ISIS-2 resulted in 25 lives saved per 1000 patients treated as well as preventing 10 nonfatal re-infarctions and 3 strokes per 1000 patients treated.50 These findings appear to be durable at 10 years of follow-up, making the duration of aspirin therapy in this setting indefinite.51 Aspirin resistance in patients with stable cardiovascular conditions occurs at a frequency of approximately 5% and confers a significant risk of death, MI, or stroke compared with those with aspirin sensitivity; however, widespread screening remains a controversial issue.52