Thromboembolism is a severe complication in atrial fibrillation. This overview presents thromboembolic disease as a single entity, ranging from stroke through mesenteric ischemia to acute limb ischemia. The PubMed, Embase, and Cochrane databases were systematically searched for the terms “atrial fibrillation” and “thromboembolism” in reports published from January 1986 to September 2009. The information of 10 evidence-based practice guideline documents and 61 further sources was systematically extracted. In atrial fibrillation, the average annual stroke risk is increased by 2.3% (lethality 30%). The annual incidence of acute mesenteric ischemia is 0.14% (lethality 70%), and that of acute limb ischemia is 0.4% (lethality 16%). In total, approximately 80% of embolism-related deaths are from stroke and 20% from other systemic thromboembolism. The ischemic symptoms generally have an acute onset but may mimic other diseases, particularly in mesenteric ischemia. Early diagnosis and treatment can limit or even prevent tissue infarction. Guideline-recommended therapy with aspirin or warfarin reduces the thromboembolic risk. Suitable patients may optimize their warfarin therapy by self-monitoring of the international normalized ratio (INR). New oral and parenteral anticoagulants with more stable pharmacokinetics are being developed. In conclusion, atrial fibrillation predisposes to thromboembolism. If ischemic stroke or systemic thromboembolism occurs, early diagnosis and treatment can improve outcomes. The thromboembolic risks are reduced by guideline-adherent antithrombotic therapy with warfarin or aspirin. Future directions may include self-monitoring of the international normalized ratio and novel anticoagulants.
Nonvalvular atrial fibrillation (AF) is the most common cardiac arrhythmia, with an overall prevalence of about 1%. The prevalence increases with age and coexisting cardiopulmonary disease. AF predisposes to thrombus formation, especially in the left atrial appendage, with risk for subsequent thromboembolism. Ischemic stroke is the predominant complication, but extracerebral thromboembolism also contributes to the elevated morbidity and mortality in AF. Early diagnosis within a few hours is crucial for individual therapy and outcome. This overview presents thromboembolism in AF as a single entity, ranging from stroke through mesenteric ischemia to acute limb ischemia.
Methods
Identification of published research
The PubMed, Embase, and Cochrane databases were searched for the Medical Subject Headings “(atrial fibrillation) AND (thromboembolism OR embolism OR ischemia OR transient ischemic attack OR stroke)” and related terms in reports published from January 1986 to September 2009, with a focus on more recent publications. Further literature was identified from the reference lists of retrieved reports. This systematic term-driven literature search was intended to find the most reports with direct linkages to the topic. Some related issues were covered by additional specific literature searches, for example, about the relevance of rehabilitation after stroke. In total, 16,409 items were retrieved.
Selection of published research
We extracted theme-specific information from 10 evidence-based practice guideline documents. Structured summary data for most of these guidelines are available from the National Guideline Clearinghouse ( http://www.guideline.gov ). Of the 16,409 retrieved items, 16,169 were excluded by screening titles and abstracts, and 104 items were excluded by reading the full text. The remaining 136 reports were considered eligible, including those with similar or overlapping contents. By comparison, 71 references were selected by consensus with the following rank order: practice guidelines (10 sources), meta-analyses or reviews (21 sources), prospective studies (18 sources), and retrospective studies (22 sources). The literature selection was limited to English-language reports. Ninety-two percent (65 of 71) of the included references were electronically available in the Portable Document Format (Adobe Systems, Inc., Mountain View, California). One textbook was added to the references.
Evidence synthesis
This overview presents thromboembolism in AF in a systematic order from its atrial origin to the peripheral end points, including incidence, symptoms, laboratory and imaging findings, therapy, and outcomes of the different organ manifestations. The evidence was synthesized by expert consensus, a standard method that is also applied for constructing parts of the guidelines ( http://www.guideline.gov ). Numerical meta-analytic methods for primary sources could not be applied for this overview, because the guidelines are not primary sources and because of the diversity of included sources (guidelines, reviews, etc.). After reading the full text, the published evidence was extracted from the Portable Document Format documents using the search function of Adobe Reader version 9 (Adobe Systems, Inc.) to assess the retrieved electronic literature as systematically as possible. For example, searching for “mesenteric ischemia” or “intestinal ischemia” and related terms yielded 301 text passages in the electronic documents. The 6 additional printed articles and textbook were similarly hand-searched. The retrieved published evidence was weighed with the same rank order as during the literature selection (evidence-based guidelines>meta-analyses or reviews>prospective studies>retrospective studies). The current levels of evidence are extensively documented in the cited guidelines. This overview does not present the pathogenesis and treatment strategies of AF. The main results of this overview are summarized by key points ( Table 1 ).
|
Results
AF
The pathogenesis and treatment strategies of AF, such as rate and rhythm control, are covered by other guidelines and publications. These treatment strategies, as well as the prevention of AF itself, can contribute to an overall reduction in the risk for thromboembolism. However, thromboembolism may occur even with optimal AF treatment.
Left atrial thrombus formation
During episodes of AF, cardiac blood flow is reduced, especially in the left atrial appendage, which is the major site of thrombus formation. Such thrombus formation is thought to be triggered by Virchow’s triad of stasis, endothelial dysfunction, and a hypercoagulable state. Thrombus sizes range from few millimeters to about 4 cm. Left atrial thrombi are found in about 5% to 14% of patients, if AF lasts >2 days. Atrial thrombi are usually diagnosed by transesophageal echocardiography. Alternative methods are cardiac magnetic resonance imaging and computed tomography. The main differential diagnosis of a left atrial thrombus is atrial myxoma. However, both entities may occur simultaneously when a thrombus is attached to a myxoma. Atrial thrombi may be dissolved by endogenous thrombolysis. Anticoagulation with warfarin helped to resolve about 75% of atrial thrombi within a month of treatment in the Assessment of Cardioversion Using Transesophageal Echocardiography (ACUTE) trial. If atrial thrombi do not resolve, they may become detached and embolize in the peripheral arterial system.
Thromboembolism
Thromboembolism most frequently occurs during episodes of actual AF or within the first 10 days after electrical, pharmacologic, or spontaneous conversion to sinus rhythm. Most events manifest in the brain, which is particularly vulnerable to ischemia, but thromboembolism in other organ systems also contributes to overall morbidity and mortality.
Ischemic stroke and transient ischemic attacks
Definition
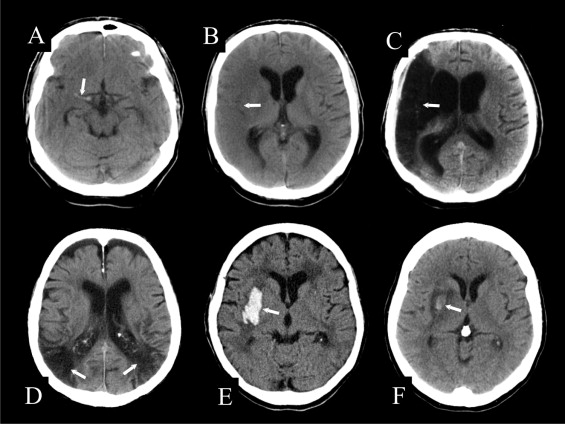
AF is associated with a high risk for cerebral thromboembolism. Clinically, 2 courses of subsequent focal cerebral ischemia are differentiated: in transient ischemic attacks, the neurologic deficit lasts on average no more than 12 minutes and is by definition completely reversible in <24 hours. In ischemic stroke, the focal neurologic deficit persists because of irreversible ischemia followed by cerebral infarction. For differential diagnosis, 3 types of focal cerebral ischemia can be differentiated: (1) local arterial thrombosis that often causes small lacunar infarcts; (2) the embolic occlusion of a main cerebral artery or its branches, which may cause partial or total infarction of the depending vascular territory ( Figure 1 ); and (3) nonocclusive watershed ischemia at the boundary between 2 adjacent vascular territories, which occurs during states of global cerebral hypoperfusion ( Figure 1 ). Cardiogenic thromboembolism frequently causes territorial infarction but may also result in clinically occult lacunar lesions.
Epidemiology
In AF, the annual stroke incidence increases with age from about 1.3% in 50- to 59-year-old patients to 5.1% in octogenarians. On average, the thromboembolism-related extra stroke risk is about 2.3% per year. With a 1-year lethality of about 30%, the associated extra mortality is about 0.7% per year. AF alone is associated with a three- to fourfold increase in stroke incidence, when statistically adjusting for other risk factors. The coincidence of AF and further factors increases the individual stroke risk accordingly. Several scores have been proposed to assess individual stroke risk. Current guidelines recommend the CHADS 2 score, which is compiled by assigning 1 point each for congestive heart failure, hypertension, age ≥75 years, and diabetes mellitus and by assigning 2 points for any history of stroke or transient ischemic attack. Additional known risk factors are smoking, hyperlipidemia, carotid artery stenosis, and other cardiovascular factors.
Clinical Symptoms
The diagnosis of transient ischemic attack and ischemic stroke is based predominately on clinical findings. The leading symptom is the acute onset of a painless, focal neurologic deficit. The extent of neurologic dysfunction is determined largely by the cerebral area affected and only in part by the size of the infarct. Transient ischemic attacks and stroke are linked with circumscribed symptoms. Classic supratentorial stroke symptoms include contralateral hemiparesis, homonymous hemianopsia, aphasia in stroke of the dominant hemisphere, and anosognosia in stroke of the nondominant hemisphere. Cerebellar stroke mainly causes ipsilateral ataxia. Brainstem ischemia usually has crossed peripheral symptoms due to the simultaneous involvement of cranial nerve nuclei and nerve tracts.
Imaging
The major task of unenhanced computed tomography or any other initial imaging is to rule out intracranial hemorrhage rather than to prove the clinical diagnosis of stroke. About 2 hours after the onset of stroke symptoms, early signs may be shown on unenhanced computed tomography, but these signs have no clear prognostic value. For example, a hyperdense middle cerebral artery indicates occlusion by a locally formed thrombus or embolus impaction ( Figure 1 ). Slight parenchymal hypodensity indicates increased water content in affected brain matter due to developing cytotoxic edema ( Figure 1 ). Within the affected territory, diffusion-perfusion-weighted magnetic resonance imaging frequently demonstrates a nonviable infarct core and a surrounding penumbra of potentially viable but hypoperfused tissue-at-risk. Contrast-enhanced computed tomographic perfusion techniques are performed for the same purpose. Changes during the temporal course of stroke are usually examined using unenhanced computed tomography. This allows the assessment of complications such as critically space-occupying edema and hemorrhagic transformation of an infarct ( Figure 1 ). The latter may range from asymptomatic petechial bleeding to focal secondary hemorrhage with mass effect, occasionally necessitating neurosurgical intervention.
Differential Diagnosis
Most stroke patients have ischemic insults, but about 15% have primary intracranial hemorrhages ( Figure 1 ). These stroke entities may be clinically indistinguishable, necessitating the routine use of imaging to rule out a hemorrhagic origin. Further differential diagnoses of stroke include complicated migraine, transient paresis after seizure, and intracranial neoplasms that may bleed or excite profound perifocal edema. Epidemiologic data indicate that strokes in patients with AF are frequently caused by cardioembolism. However, because of co-morbidities, these patients also encounter stroke due to coexisting diseases, such as carotid artery disease or arterial hypertension.
Therapy
Public education programs aim to increase public awareness of stroke symptoms to decrease referral time to stroke units. Guidelines provide general recommendations for stroke therapy. Individualized therapeutic decisions are based on a patient’s clinical findings and co-morbidities. Supportive therapy aims at limiting cerebral infarction. This includes the prevention of fever, hyperglycemia, and malignant hypertension. Specific therapy with systemic intravenous thrombolysis using recombinant tissue plasminogen activator (rtPA) is given to selected patients with strokes lasting <3 hours. Different studies have investigated further diversifications of stroke treatments, including thrombolysis for stroke duration beyond the 3-hour time window, the imaging-based selection of patients who are more likely to benefit from thrombolysis, and the local intra-arterial administration of thrombolytic agents.
Outcome
In AF, about 30% of patients with strokes die within 1 year. From 15% to 30% of stroke survivors remain permanently disabled. Cardioembolic strokes are, on average, more disabling and carry higher mortality than noncardioembolic strokes. In patients with strokes who require mechanical ventilation, prognoses are generally poor, but a small number survive without severe disability. There is evidence that stroke survivors benefit from rehabilitation.
Splenic thromboembolic infarction
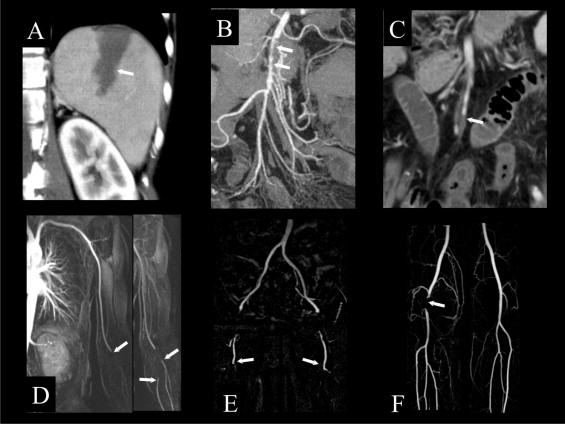
Splenic thromboembolic infarction is a rare finding in AF. Because of an unspecific or even quiescent presentation, only 10% of splenic infarctions are clinically suspected. Symptoms and laboratory findings may include left upper abdominal pain, fever, and leukocytosis. Contrast-enhanced computed tomography shows splenic hypodensity during the venous equilibrium phase. Classically, this hypodense lesion is wedge-shaped, indicating its segmental vascular origin ( Figure 2 ). Other causes of splenic infarction include hematologic disorders, thromboembolism from other cardioarterial sources, and endocarditis with septic embolism and splenic inflammation. Rarely, splenic infarction progresses to massive subcapsular hemorrhage or splenic abscess, necessitating laparotomy and splenectomy. However, the usual course of splenic infarction is asymptomatic or with gradual resolution of pain without clinical sequelae.
Acute renal thromboembolism
In AF, a low incidence of about 0.01% is reported for renal thromboembolism, but the condition may be underdiagnosed. Most patients present with acute flank pain or generalized abdominal pain, sometimes associated with fever and vomiting. The clinical findings are frequently unspecific. Therefore, the standard differential diagnosis encompasses other causes of acute abdominal pain, including urolithiasis, pyelonephritis, and acute mesenteric ischemia. Typical laboratory findings of renal ischemia are an elevated serum lactate dehydrogenase (LDH) level >400 U/dl and leukocytosis. Microhematuria and proteinuria are present in about 50% of patients. Renal function is frequently impaired, with serum creatinine levels increasing to >2 mg/dl during the course of the disease. Catheter angiography is the gold standard of imaging in renal artery stenosis and occlusion, with sensitivity reported as high as 100%. Renal scintigraphy has similarly high sensitivity for diagnosing renal ischemia. Computed tomography has intermediate sensitivity of about 80% for the detection of thromboembolic arterial occlusion, whereas ultrasound has low sensitivity. Conservative therapy includes anticoagulation with heparin. Systemic or local transcatheter thrombolysis has been performed with variable effect and carries a risk for hemorrhage. In a retrospective study of 44 patients, about 25% had persistent latent or apparent renal failure, with serum creatinine levels >2 mg/dl, while renal function normalized in 60%. Eleven percent of patients died because of further complications.
Acute thromboembolic mesenteric ischemia
Definition
Embolic obstruction of the superior mesenteric artery or its branches can cause acute ischemia of the small intestine and proximal colon. The embolus often lodges just proximal to the origin of the middle colic artery, so that the jejunum remains perfused. Embolic occlusion of the inferior mesenteric artery may cause ischemic colitis of the distal colon if the normal collateral arterial supply through the arc of Riolan and via anorectal arteries is insufficient.
Epidemiology
In AF, acute thromboembolic mesenteric ischemia has a low incidence of about 0.14%/year. However, the condition has a high lethality of approximately 70%. This causes an additional mortality of about 0.1% annually.
Clinical Symptoms
Patients with acute intestinal ischemia present with severe nonremitting abdominal pain that is initially out of proportion to the findings on physical examination. This is often followed by an interval of several hours with only a few symptoms. Finally, bowel necrosis and perforation lead to peritonitis and shock.
Laboratory and Imaging Findings
Laboratory evaluation often shows mild leukocytosis and metabolic acidosis, with lactic acidosis in the initial stage. As the illness progresses to bowel necrosis and perforation, laboratory markers of inflammation increase. Abdominal x-rays may initially be normal or show dilated loops of intestine with air-fluid levels, indicating the onset of paralytic ileus. To consider differential diagnoses in acute abdominal pain of unknown origin, contrast-enhanced computed tomography is the diagnostic modality of choice. In acute thromboembolic mesenteric ischemia, high-resolution computed tomographic angiography can show the site of arterial occlusion ( Figure 2 ). With time, there is wall thickening of the affected ischemic bowel segments. The progression of intestinal ischemia to necrosis is indicated by intestinal pneumatosis, perforation, and peritonitis. If noninvasive computed tomographic angiography does not establish the diagnosis, invasive catheter angiography can show the mesenteric occlusion with the highest image resolution. Alternatively, explorative laparotomy is performed.
Differential Diagnosis
Acute abdomen due to acute mesenteric thromboembolism has several differential diagnoses, including rupture of an aortic aneurysm, abdominal inflammatory diseases, and diabetic ketoacidosis with pseudoperitonitis. Most similar is the preexisting atherosclerosis of the superior mesenteric artery with superimposed acute local thrombosis. A further vascular differential diagnosis is nonocclusive mesenteric ischemia due to blood flow redistribution away from the bowel towards vital organs. This may occur with low cardiac output states, shock, catecholamines, or the administration of other vasoconstricting drugs. A further vascular differential diagnosis is mesenteric or portal venous thrombosis, which can be imaged by biphasic contrast-enhanced computed tomography with scanning in the arterial and venous perfusion phases.
Therapy
Selected patients with superior mesenteric thromboembolism without signs of bowel necrosis can be treated by local transcatheter thrombolysis, balloon angioplasty, and, occasionally, stenting. Patients treated in this way may still require laparotomy, which is the therapeutic gold standard in embolic mesenteric ischemia. It consists of embolectomy, eventually bypass grafting, and the resection of nonviable bowel. When appropriate, a second-look laparotomy is performed 24 to 48 hours after revascularization to reevaluate intestinal viability.
Outcome
If acute occlusive mesenteric ischemia is diagnosed and treated within the first 6 hours, bowel resection can often be avoided, and the general prognosis is good. Treatment thereafter frequently requires the resection of necrotic bowel loops, and lethality increases progressively with time. Frequently, acute occlusive mesenteric ischemia remains unrecognized initially, so that specific treatment is delayed for ≥24 hours. This results in a high overall lethality of about 70%. Survivors treated by small bowel resection may have short bowel syndrome.
Acute thromboembolic limb ischemia
Definition
In acute thromboembolic limb ischemia, the affected artery is suddenly occluded by an embolus from a cardiac or other upstream source. Arterial emboli typically lodge at branch points in the arterial tree, where the arterial lumen diminishes. Occasionally, a large embolus is stopped at the aortic bifurcation, forming a saddle embolus that extends into both iliac arteries. Thrombus fragments may then cause bilateral acute leg ischemia, which has particularly high lethality ( Figure 2 ). However, more commonly, acute thromboembolism affects only 1 extremity.
Epidemiology
Patients with AF have an additional incidence of aortoiliac and lower-extremity arterial thromboembolism of about 0.4%/year. The upper limbs are less frequently affected than the lower limbs. The associated lethality at 12 months is about 16%, resulting in an estimated extra annual mortality of about 0.06%.
Clinical Symptoms
Acute limb ischemia is characterized by an acute onset of pain, paralysis, paresthesia, pulselessness, and paleness. Because of superficial collaterals, reduction of skin temperature usually begins a limb segment below the level of the arterial occlusion. The severity of symptoms varies considerably among patients, depending on the extent of arterial occlusion as well as the volume of collateral perfusion.
Laboratory and Imaging Findings
Acute limb ischemia has no specific laboratory findings. Duplex sonography helps to confirm the clinical diagnosis and often demonstrates the level of the occlusion. However, catheter-based digital subtraction angiography is the gold standard for the imaging of peripheral arterial disease, including acute limb ischemia. Digital subtraction angiography has the highest image quality of all angiographic techniques but may have complications due to its invasive nature. Noninvasive imaging alternatives are computed tomography and magnetic resonance angiography. Generally, computed tomographic angiography can depict pelvic and leg arteries with good image quality, although in the lower leg extensive atherosclerosis may hinder differentiation between contrast-enhanced residual lumen and calcified obstruction. Magnetic resonance angiography tends to slightly overestimate arterial stenoses but shows occlusions well and is not affected by vascular calcification ( Figure 2 ).
Differential Diagnosis
In patients with AF and coexisting atherosclerosis, acute limb ischemia may be caused either by systemic embolism or by local arterial thrombosis, which may be difficult to distinguish. Further differential diagnoses of acute limb ischemia include arterial occlusion due to trauma and popliteal artery entrapment.
Therapy
Therapy for acute thromboembolic limb ischemia depends on individual findings. Interventional transcatheter approaches include local arterial thrombolysis with fibrinolytic agents, thrombus aspiration, and the use of thrombectomy devices. Patients with severe limb ischemia may require immediate surgical embolectomy or revascularization to prevent limb necrosis, rhabdomyolysis, and Tourniquet’s toxic reperfusion syndrome. If ischemia is irreversible, amputation is performed.
Outcome
In the Rochester randomized prospective trial, the limb loss rate at 12 months was about 18% with the surgical and thrombolytic approaches. Lethality at 12 months was about 16% with thrombolysis and 42% for surgical patients. The survival difference was attributable primarily to more major in-hospital complications in the surgical group.
Prevention of thromboembolism
Guidelines
In AF, the treatment of thromboembolic complications generally aims at limiting tissue infarction and restoring perfusion to the tissue-at-risk. However, the major goal is to prevent such thromboembolism. For this purpose, two current guidelines recommend antithrombotic therapy with aspirin or warfarin after the assessment of individual risk ( Table 2 ). The American College of Cardiology, American Heart Association, and European Society of Cardiology (ACC/AHA/ESC) 2006 guidelines incorporate the CHADS 2 score–based recommendations from the American Heart Association and American Stroke Association (AHA/ASA) 2006 guidelines and add some cardiovascular risk factors ( Table 2 ): (1) patients with nonvalvular AF and low stroke risk should receive the antiplatelet drug aspirin, and (2) patients with ≥2 moderate-risk factors or with any high-risk factor should receive oral anticoagulation with the vitamin K antagonist warfarin, unless contraindicated ( Table 2 ). These recommendations have also been incorporated in the American College of Chest Physicians (ACCP) 2008 guidelines.
| Risk Category (ACC/AHA/ESC 2006) | CHADS 2 Score † (AHA/ASA 2006) | Recommended Therapy |
|---|---|---|
| No risk factors | 0 | Aspirin (81–325 mg/d) |
| One moderate-risk factor | 1 | Aspirin (81–325 mg/d) or warfarin (INR 2–3, target 2.5) |
| ≥2 moderate-risk factors or any high-risk factor | ≥2 | Warfarin (INR 2–3, target 2.5) ‡ |
| Less validated or weaker risk factors | Moderate-risk factors | High-risk factors |
|---|---|---|
| Coronary artery disease | LVEF ≤35% | Prosthetic heart valve ‡ |
| Thyrotoxicosis | C ongestive heart failure | Mitral stenosis |
| Age 65–74 years | H ypertension | Previous systemic embolism |
| Female gender | A ge ≥75 years | Previous TIA |
| D iabetes mellitus | Previous S troke |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


