Type
Aetiology
Primary spontaneous
No underlying lung disease (but blebs/bullae commonly present)
Secondary spontaneous
Associated with underlying lung disease, e.g. COPD, Cystic Fibrosis, AIDS
Traumatic
Related to trauma to the thorax
Iatrogenic
Secondary to transthoracic or transbronchial lung biopsy (10 %), central venous catheterisation, supraclavicular nerve block
13.3 Pathology
Spontaneous pneumothorax, both primary and secondary, has been shown to be associated with the presence of pleural blebs/bullae – also termed ‘emphysema like changes’ (Figs. 13.1, 13.2, and 13.3). Blebs/bullae are visible on CT scanning in 89 % of patients with PSP (Mitlehner et al. 1992), in 90 % of cases at visual inspection using video-assisted thoracoscopy (Mouroux et al. 1996) and up to 100 % of cases at thoracotomy (Donohue et al. 1993). It has long been assumed that the cause of spontaneous pneumothorax is rupture of these blebs/bullae (Light 2001). Despite this widely held opinion, there is no direct evidence which confirms rupture of blebs/bullae as the cause of SP. An Electron microscopy study of resected tissue has failed to confirm the presence of rupture in any blebs/bullae (Ohata et al. 1980). More recently, an elegant study using fluorescein-enhanced autofluorescence at thoracoscopy to study the visceral pleura of patients with spontaneous pneumothorax has shown that patients who have spontaneous pneumothorax have evidence of diffuse pleural abnormalities at sites not associated with pleural blebs/bullae. Interestingly this study noted areas of air leak in a small proportion of patients. None of the observed air leaks were directly associated with pleural bullae (Noppen et al. 2006).
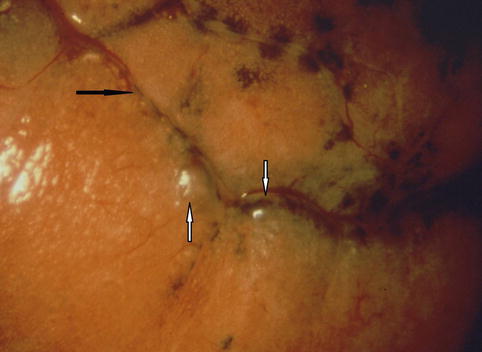
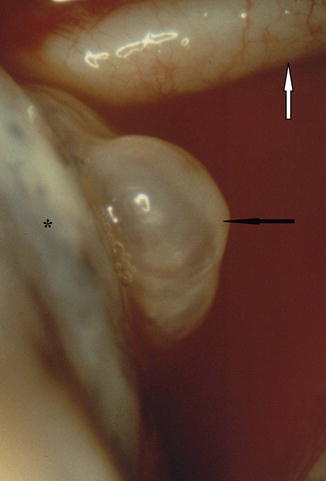
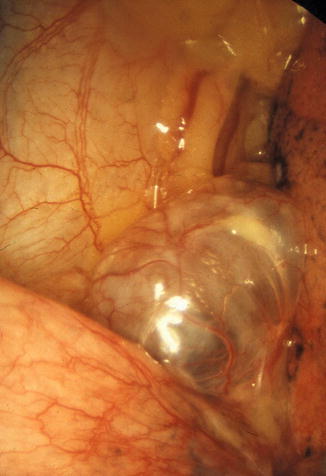

Fig. 13.1
Small blebs in spontaneous pneumothorax. The blebs (white arrows), close to the fissure (black arrow) and are visible at thoracoscopy with high-quality optical magnification. Anthracotic deposits are located on the surface of the lung (Courtesy C Boutin and Ph Astoul, Marseille, France)

Fig. 13.2
Blebs (black arrow) on the apical surface of the lung. This translucent and fragile blister has a very thin, nonvascularised wall and is very frequently found in patient with primary spontaneous pneumothorax. In this case it is very close to the subclavian artery (white arrow) (Courtesy C Boutin and Ph Astoul, Marseille, France)

Fig. 13.3
Emphysematous bullae with a vascularised surface and a size usually superior than 2 cm in diameter. In this case, the bullae are inflated and therefore it is not the cause of the spontaneous pneumothorax (Courtesy Ph Astoul, Marseille, France)
PSP has been shown to be associated with an inflammatory process within the lung, with increased numbers of inflammatory cells, particularly macrophages present in bronchoalveolar lavage (Schramel et al. 1995), and the histology of resected lung reveals the presence of an obstructive bronchiolitis (Lichter et al. 1971). Furthermore, the risk of recurrence cannot be predicted by the presence of blebs/bullae either at thoracoscopy (Janssen et al. 1995) or on CT imaging (Martinez-Ramos et al. 2007). It has been hypothesised that the site of air leak is actually distant to the blebs/bullae and related to increased distal airways pressures consequent to obstructive bronchiolitis (Noppen et al. 2002b). It is important to bear in mind that the site of air leak is unlikely to be solely from the blebs/bullae when we later consider recurrence prevention techniques (Fig. 13.4).
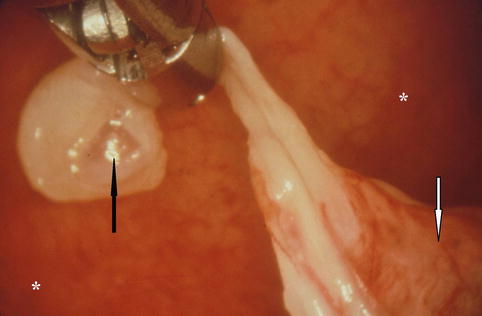

Fig. 13.4
Spontaneous pneumothorax due to the rupture (black arrow) of a large emphysematous bullae on the lung apex (white arrow). However the site of air leak is unlikely to be solely from the blebs/bullae when we consider recurrence prevention techniques (* parietal pleura) (Courtesy C Boutin and Ph Astoul, Marseille, France)
13.4 Management of Spontaneous Pneumothorax
13.4.1 Initial Treatment
There are a number of published guidelines which make recommendations regarding the management of both primary and secondary spontaneous pneumothorax (Henry et al. 2003; Baumann et al. 2001). There are differences between the guidelines which highlight the fact that the optimal management strategy for spontaneous pneumothorax is yet to be defined. Detailed discussion of the initial management of spontaneous pneumothorax is beyond the scope of this chapter but includes observation, pleural aspiration and intercostal drainage. The choice of initial treatment option depends upon the size of the pneumothorax, the physiological impact on the patient and whether there is underlying pulmonary disease (Marquette et al. 2006). Small pneumothoraces which have minimal physiological impact on the patient can safely be observed without invasive intervention. If removal of air is warranted, then the initial approach should be simple aspiration (Astoul 2010). This has been conclusively shown in randomised controlled trials to be equally effective as intercostal drain insertion with a success rate of approximately 80 % (Noppen et al. 2003; Andrivet et al. 1995; Ayed et al. 2006; Harvey and Prescott 1994). If aspiration is unsuccessful or the patient is severely compromised, then an intercostal drain should be inserted. The presence of underlying pulmonary disease lowers the threshold for consideration of insertion of an intercostal drain rather than simple aspiration. Accurate estimation of the size of a pneumothorax on a plain chest radiograph can be difficult but for clinical purposes is guided by the distance of the lung edge from the cupola/chest wall. A distance of >2 cm should be regarded as significant.
13.4.2 Recurrence Prevention
Spontaneous pneumothorax is associated with a significant recurrence rate. After a first primary spontaneous pneumothorax treated without any form of pleurodesis, the recurrence rate is approximately 30 % with most recurrences occurring in the first 2 years (Schramel et al. 1997). The recurrence rate increases significantly after a second and third episode to 62 and 83 % respectively (Gobbel et al. 1963). Independent risk factors for recurrence of a primary spontaneous pneumothorax include ongoing cigarette smoking, increasing age and height (Lippert et al. 1991). The risk of recurrence after a secondary spontaneous pneumothorax is higher than that for a primary spontaneous pneumothorax (Lippert et al. 1991) approaching 40–50 % with similar independent risk factors to PSP. Importantly, the presence of blebs/bullae either ipsilaterally or contralaterally is not predictive of recurrence (Martinez-Ramos et al. 2007) and hence should not be used to guide management decisions. Given the risk of recurrence, particularly after a second PSP, recurrence prevention is an important aspect of the management of spontaneous pneumothorax.
Procedures available for recurrence prevention include pleurodesis through an intercostal drain with talc (Almind et al. 1989), medical thoracoscopy with talc poudrage and surgical intervention with a combination of treatment of blebs/bullae (bullectomy/wedge resection/stapling of bullae) and a form of pleurodesis either chemical or with abrasion or subtotal pleurectomy. The surgical interventions can be carried out through video-assisted thoracic surgery (VATS) or via thoracotomy. Table 13.2 summarises the reported efficacy of these different approaches.
Table 13.2
Different approaches for the management of recurrent spontaneous pneumothorax
Reference | Technique | Procedure | PSP/SSP/mixed | Success rate (%) |
|---|---|---|---|---|
Athanassiadi et al. (1998) | Thoracotomy | Stapling of bullae + pleural abrasion | Mixed | 99 |
Korner et al. (1996) | Thoracotomy | Wedge resection | Mixed | 95 |
Ayed and Raghunathan (2000) | VATS | Stapling of bullae + pleural abrasion | Mixed | 95 |
Lang-Lazdunski (2003) | VATS | Bleb excision/pleural abrasion | PSP | 97 |
Maskell et al. (2004) | MT | TP | SSP | 95 |
Tschopp et al. (1997) | MT | TP | Mixed | 95 |
Tschopp et al. (2002) | MT | TP | PSP | 95 |
Pleurodesis through an intercostal drain is less effective than other treatment options with a recurrence rate of 8–13 % depending on the pleurodesing agent used – with talc shown to be the most effective agent (Almind et al. 1989). However this technique should be considered for those either unwilling or unfit to undergo more invasive procedures (Kennedy et al. 1995). The reason for the lower efficacy of pleurodesis without visualisation of the pleura (slurry) may be related to the inability to confirm diffuse spread of the pleurodesing agent.
For all other patients, a more invasive approach should be taken. There are proponents for both surgical intervention and medical thoracoscopy. A dearth of randomised controlled trials comparing the different approaches makes it difficult to say with certainty what the best approach is. The ‘gold standard’ in terms of recurrence prevention remains intervention via thoracotomy (Henry et al. 2003) as this has been shown to have a very high success rate approaching 100 % in some series (Nkere et al. 1994; Athanassiadi et al. 1998), but most surgeons prefer VATS (Baumann et al. 2001) which is associated with less cost and has lower rates of morbidity and mortality (Crisci et al. 1996). VATS procedures are much more commonly performed in the current era compared to thoracotomy. VATS procedures are acknowledged to have a slightly lower success rate with a long-term recurrence rate of approximately 5 % (Ayed and Raghunathan 2000) and a relative risk of recurrence of 4.7 when compared to thoracotomy (Barker et al. 2007), but this is balanced by the lower morbidity/mortality.
There are two key differences between a surgical approach (VATS or thoracotomy) and medical thoracoscopy. The first is that surgical therapies require a general anaesthetic with its inherent risks and secondly most surgeons will carry out both pleurodesis of some form and a treatment directed at removing/repairing any blebs/bullae. As discussed earlier, there is no evidence to support the view that blebs/bullae are the sole site of air leak and indeed there is a body of evidence to support the fact that they are not the source of air leak. Although there has been no randomised controlled trial comparing pleurodesis + surgical repair of blebs/bullae with pleurodesis alone, a number of surgical studies have clearly shown that the risk of recurrence of pneumothorax is significantly higher if some form of pleurodesis is not carried out irrespective of the other surgical interventions performed (Hatz et al. 2000; Liu et al. 1995; Korner et al. 1996).
The timing of intervention is a further area of debate. There is a broad consensus that any patient presenting with a recurrent or contralateral pneumothorax, be it primary or secondary, should be offered some form of recurrence prevention as the risk of a further pneumothorax after a first recurrence is significantly higher than after a first event (Gobbel et al. 1963). Some guidelines recommend recurrence prevention for all secondary spontaneous pneumothoraces on the grounds that the clinical impact of a pneumothorax on a patient with underlying lung disease is much greater (Baumann et al. 2001). The area of greatest debate is whether or not to offer recurrence prevention to patients after a first PSP. In this situation we have to balance the risk of recurrence (approximately 30 %) against the risk of carrying out invasive pleural procedures. It has been shown that carrying out VATS on first time PSP is a cost-effective strategy as compared with conservative treatment, as the costs of treating recurrent pneumothoraces are significant (Schramel et al. 1996). These authors recognised that the cost of medical thoracoscopy and talc poudrage is 62 % less than VATS and would be even more cost-effective. This is supported by similar evidence from other groups (Tschopp et al. 2002).
13.5 The Role of Medical Thoracoscopy in the Management of Spontaneous Pneumothorax
Medical thoracoscopy and talc poudrage has been employed, with good effect, as a method of recurrence prevention for both primary and secondary spontaneous pneumothorax for over three decades (Guerin and Boutin 1999). More invasive procedures such as pleurectomy or bullectomy cannot be performed without either general anaesthesia with single-lung ventilation or epidural anaesthesia. We discuss in detail here the supporting evidence for and the role of medical thoracoscopy–talc poudrage (MT–TP) in the management of both primary and secondary pneumothorax and follow on from this with a discussion of the technical aspects of talc poudrage via medical thoracoscopy for pneumothorax (Campos et al. 1997; Ferrer et al. 2001).
13.5.1 Primary Spontaneous Pneumothorax
Given that the aetiology of primary spontaneous pneumothorax has never been shown to be directly related to rupture of bullae and that the pleura of patients with spontaneous pneumothorax has been shown to be diffusely abnormal at areas distant to any bullae or blebs, the requirement for anything more than effective pleurodesis can be debated. It has been shown that a diffuse form of pleurodesis, i.e. talc poudrage, is more effective than a local form of pleurodesis, i.e. subtotal pleurectomy (Bresticker et al. 1993; Cardillo et al. 2006). Talc poudrage was first described in 1935 and has been used successfully as a sole method of recurrence prevention by cardiothoracic surgeons carrying out thoracoscopy under general anaesthetic (Nandi 1980; van de Brekel 1993). The early case series reported in the medical literature for MT–TP reported on its use for either complicated pneumothorax (i.e. prolonged air leak) or recurrent pneumothorax. These series treated both primary and secondary pneumothoraces. They reported good long-term efficacy with recurrence rates of 7 % (Guerin and Boutin 1999; El Khawand 1995). More recently a prospective study evaluating the safety and efficacy of MT–TP for both complicated pneumothorax and recurrent pneumothorax showed MT–TP to be a safe and effective method of recurrence prevention (Tschopp et al. 1997). This study recruited 89 patients, and after a mean follow-up of 5.1 years, there was a recurrence rate of 5 %, which are on a par with the efficacy rates quoted for VATS procedures for pneumothorax recurrence prevention (Ayed and Raghunathan 2000). This study did note that recurrence of pneumothorax was significantly more likely in the presence of bullae greater than 2 cm in diameter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


