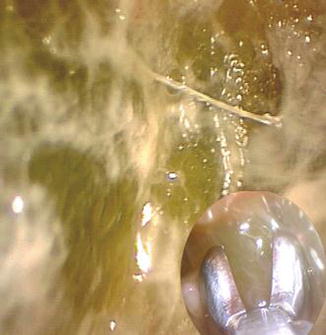
Fig. 16.1
A typical empyematous collection
Exploration of the pleural cavity to identify fibrin membranes (Fig. 16.2) and to detect loculations (Fig. 16.3), as well as possible neoplastic nodules, or intrapleural bubbling which may be an indirect sign of fistula (Fig. 16.4).
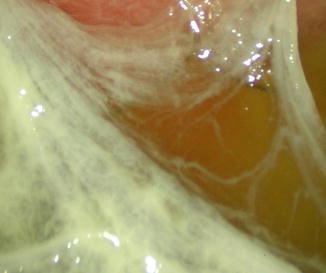
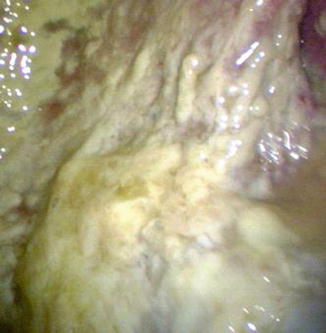
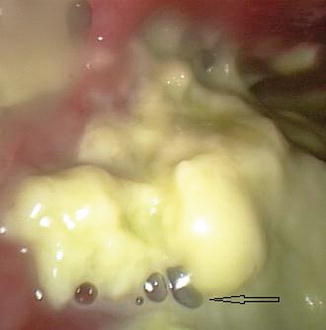

Fig. 16.2
Fibrin membranes in the pleural cavity

Fig. 16.3
An empyematous loculation in the left pleural cavity

Fig. 16.4
Bubbles (arrow) indicating an associated lung parenchymal fistula
Removal of fibrinous membranes (Fig. 16.6) and purulent material from the cavity (Fig. 16.7) and, in addition, from the parietal and visceral pleural surfaces.
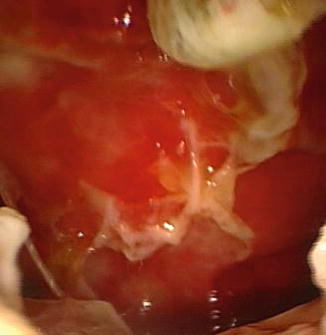
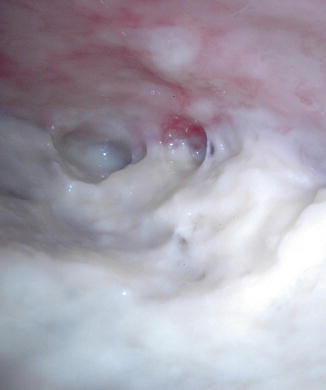

Fig. 16.6
The pleural cavity after the removal of the fibrinous membranes

Fig. 16.7
Purulent material in the pleural cavity
Pleural biopsy.
Pleural space lavage with saline solution.
Introduction of a chest tube, with a caliber large enough (>24 F) to remove dense and viscous pus and fibrin detritus. This can be performed, if necessary, under visual control (Fig. 16.8).
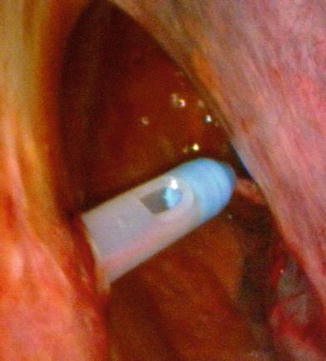

Fig. 16.8
Drain placement under visual control
16.6.3 Results
The published case studies on thoracoscopy in the infection of the pleural space (Table 16.1) deal principally with empyema and include both medical and surgical techniques, of which the latter is undoubtedly more numerous.
Table 16.1
Medical (M) and surgical thoracoscopy (S) in parapneumonic effusion and empyema
Author | Year | Patients | % of success | % of complications |
|---|---|---|---|---|
Colt (M) | 1995 | 7 | 86 | 14 |
Solèr (M) | 1997 | 16 | 73 | 0 |
Striffeler (S) | 1998 | 67 | 72 | 4 |
Reynard (M) | 2004 | 5 | 100 | 0 |
Brutsche (M) | 2005 | 127 | 91 | 9 |
Luh (S) | 2005 | 234 | 86.3 | 8.3 |
Wurnig (S) | 2006 | 130 | 91 | 9 |
Solaini (S) | 2007 | 120 | 91.8 | 11 |
In general these studies report positive results, with rates of primary success (meaning recovery without subsequent thoracotomy or conversion from thoracoscopy to thoracotomy) between 60 and 100 % and higher if the procedure was performed earlier in the clinical course. However the number of patients treated was generally small, and only a few authors present case series with more than 100 patients.
In general there is total agreement about the advantages of VATS over thoracotomy, with shorter hospitalization, lower cost, and better cosmetic results.
The medical thoracoscopy experience outline the minimal invasive nature of the method, together with lower cost compared to VATS. In addition it has advantages in high-risk anesthetic groups such as frail patients.
Complications were strictly related to the complexity of the cases treated and were represented mainly by bleeding and extensive air leaks, with reported incidence between 0 % (Sendt et al. 1995; Cassina et al. 1999) and 16 % (Angelillo Mackinlay et al. 1996). In some surgical series which included subjects with serious comorbidity, deaths did occur (Angelillo Mackinlay et al. 1996; Landreneau et al. 1996; Weissberg and Refaely 1996; Lawrence et al. 1997; Wait et al. 1997).
16.7 Conclusions
Thoracoscopy is certainly effective in the treatment of pleural infections, especially in multiloculated empyema, preventing progression to thoracotomy (Silen and Naunheim 1996), even if large randomized studies have yet to be performed to confirm this finding (Waller 2002).
Medical thoracoscopy can play an important role in patients in poor health and high surgical risk. It is an intermediate drain procedure between chest tube placement and surgical thoracoscopy (VATS) performed under general anesthesia. Moreover, it is an efficacious procedure with associated low cost. It should be performed early in the disease to try to avoid chronic evolution of the disease.
16.8 Pleural Infections – Mini-Atlas
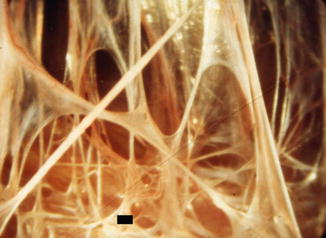
Fig. 16.9




An empyema at an early stage. There are multiple nonvascularized fibrotic adhesions, between the lung (■) and the chest wall. It is easy to severe such adhesions with the tip of optical biopsy forceps (Courtesy Ph Astoul – Marseille – France)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree