Chapter 128
Thoracic Outlet Syndrome
Venous
Louis M. Messina
Based on a chapter in the seventh edition by Andres Schanzer and Louis M. Messina
Paget–von Schroetter syndrome was defined by Hughes in 1949 when he undertook a comprehensive review of the world’s literature related to subclavian and axillary vein thrombosis.1 In 1875, Paget had defined the syndrome of acute arm swelling and pain and thought that it was due to vasospasm.2 However, in 1884, von Schroetter was the first to attribute these symptoms of acute upper extremity pain and swelling to subclavian and axillary vein thrombosis.3
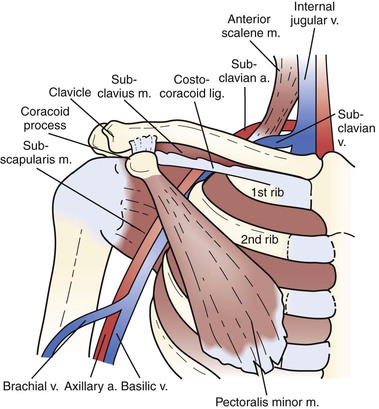
Subsequent to this publication, primary subclavian-axillary vein thrombosis was most often identified in individuals who participated in activities that involve repetitive, vigorous exertion of the upper extremity. Primary subclavian-axillary vein thrombosis is common in young athletes and people employed in occupations that require repetitive activities with the arms elevated. Thus, in otherwise healthy individuals, subclavian venous compression occurs intermittently during strenuous activity, usually with the arm positioned in an elevated position. In such circumstances, the repetitive external compression of the vein occurs between the clavicle and the underlying subclavius muscle and fibers of the costocoracoid ligament from above and by the first rib and the anterior scalene muscle inserting on the tubercle of the first rib from below (see Chapter 125). Such repetitive compression causes external vein wall compression, endothelial injury, and intermittent stasis. Eventually, perivenous and endovenous fibrosis that can lead to acute thrombosis.
Primary Subclavian-Axillary Vein Thrombosis
Epidemiology
Primary upper extremity deep venous thrombosis (DVT) is a rare disorder that occurs in 2 per 100,000 individuals per year.4 The annual incidence in the general population is 0.1%, and it increases with aging to as high as 1%.5 It is estimated that upper extremity DVT accounts for approximately 2% to 4% of all cases of DVT.5,6 Upper extremity DVT can be a relatively common occurrence in a hospital, with an estimated prevalence of 2 cases per 1000 hospital admissions.7
Etiology
Venous thoracic outlet syndrome (TOS) is a condition that ultimately results in thrombosis or severe stenosis of the subclavian-axillary vein secondary to chronic extrinsic mechanical compression. This clinical syndrome has been referred to as effort thrombosis because of its association with young, otherwise healthy individuals who engage in activities requiring repetitive arm and shoulder motion.8–11 The venous pathology is a direct result of repetitive injury to the subclavian vein at the level of the costoclavicular space, the most medial aspect of the thoracic outlet (Fig. 128-1). The key anatomic structures contributing to compression of the subclavian vein and recurrent venous trauma are the first rib, the clavicle with its associated subclavius muscle and fibrous costocoracoid ligament, and the anterior scalene muscle and tubercle.1,10,12,13 A cycle of alternating posttraumatic inflammation and quiescence leads to perivenous fibrosis, endothelial injury, stasis of blood flow, and thrombosis.12
Although venous TOS can occur in the absence of any identifiable anatomic abnormality,14,15 a diverse array of anomalies associated with the thoracic outlet has been reported at the time of surgery, including abnormalities of the anterior scalene, subclavius, pectoralis minor, and scalenus minimus muscles; bone abnormalities of the clavicle and ribs; and ligamentous abnormalities of the costocoracoid ligament.10,14–20 In many cases, compression of the subclavian-axillary vein may occur at the costoclavicular space without progression to thrombosis. This point is underscored by venographic studies evaluating the contralateral extremity in patients with confirmed subclavian-axillary vein thrombosis; although significant compression with provocative measures is visualized in 56% to 80% of contralateral limbs, the incidence of bilateral thrombosis is markedly less at 2% to 15%.9,21–27
Clinical Findings
Venous TOS usually develops in young, healthy patients with few if any comorbid conditions. The mean age at diagnosis is 32 years, with the majority of patients affected between the second and fourth decades.27 Traditionally, men have been reported to be affected more often than women; however, the largest series published to date (312 affected extremities) reported an equal gender ratio.27 Individuals who perform strenuous or sustained upper extremity activities, whether athletic or occupational, are particularly prone to the development of subclavian-axillary vein thrombosis. The dominant arm is involved in the majority of cases.26
Upper extremity edema is the hallmark characteristic associated with subclavian-axillary vein thrombosis. The edema is often but not always accompanied by pain and cyanosis of the affected extremity. The edema usually involves the shoulder, arm, and hand and is characteristically nonpitting. Dilated superficial veins over the shoulder, neck, and anterior chest wall can often be visualized as collateral veins that accommodate to the increased venous hypertension (a pattern often referred to as first rib bypass venous collaterals).12 A minority of patients may also have symptoms of neurogenic TOS (see Chapter 126). This association may be due to the presence of multiple anomalies of the thoracic outlet anatomy and performance of repetitive upper extremity arm activities.
Most patients complain of some degree of pain, which is often described as aching, stabbing, or a feeling of tightness that worsens with exertion.10 Extended use of the arm may cause an increase in arterial blood flow for which the limited collateral venous bed is unable to compensate; this leads to increased venous hypertension and subsequently to worsening symptoms of venous stasis and arm congestion. Depending on the timing of evaluation and treatment, the occlusion and resulting symptoms can be acute or chronic.
Urschel and Razzuk reported that of all patients with venous TOS in their 30-year experience (312 extremities), 93% complained of arm swelling, 77% demonstrated bluish discoloration, 66% had aching pain with exercise, and only 8% reported minimal symptoms.27 Similarly, Swinton and coauthors reported in their series that 52% of patients were symptomatic, 39% were completely disabled, and only a small proportion, 9%, were asymptomatic.28
The two most severe potential complications of subclavian-axillary vein thrombosis are pulmonary embolism and upper extremity phlegmasia cerulea dolens (venous gangrene). Fortunately, both are reported to occur very infrequently. The reported incidence of pulmonary embolism secondary to subclavian-axillary vein thrombosis is less than 12%.1,10,11,29–31 Furthermore, the small clot burden, compared with iliofemoral DVT, may reduce the clinical impact of this entity. Venous gangrene is exceedingly rare and has been limited to case reports in patients with malignant disease or an underlying hypercoagulable state.32 No reports of venous gangrene occurring secondary to venous TOS have been published.
Diagnostic Evaluation
The diagnosis of subclavian-axillary vein thrombosis requires recognition of the clinical signs and symptoms just presented, followed by definitive imaging studies.
Duplex Ultrasound
Duplex ultrasonography is the first step in confirming a clinical suspicion of venous TOS. Overlying structures such as the clavicle may make duplex interrogation of the vascular structures coursing through the thoracic outlet challenging. Therefore, duplex examinations relying on B-mode ultrasound alone have historically demonstrated low sensitivity (54%) and high specificity (100%) for the detection of subclavian-axillary vein thrombosis.33 Recent technologic advances such as color-flow duplex imaging, used in conjunction with indirect criteria suggesting the presence of an occlusion (evaluation for phasicity of flow with respiration and augmentation with compressive maneuvers), have led to markedly increased sensitivity (81% to 100%) while maintaining high specificity (82% to 100%).23,34–38 See later section, Secondary Subclavian-Axillary Vein Thrombosis, for a comprehensive review of the role of duplex scanning in this diagnosis as well as an algorithm should the results of duplex scanning be equivocal.
Magnetic Resonance and Computed Tomographic Venography
Magnetic resonance venography (MRV) is another noninvasive imaging modality that has been used with increasing frequency for the diagnosis of venous TOS.17,39–41 Although the sensitivity and specificity of MRV have been reported to be comparable to those of duplex ultrasound, I have not found this test to be necessary for patients with suspected venous TOS. The cost and time required for completion of the examination are substantial, and I have therefore continued to choose duplex ultrasound. With time and more accumulation of data, the role of MRV in patients with suspected subclavian-axillary vein thrombosis will be better defined.
Computed tomographic venography (CTV) shows high concordance with duplex ultrasound. When imaging of the extremity veins and pulmonary arteries is considered clinically indicated, CTV can be used to reliably diagnose DVT.
Venography
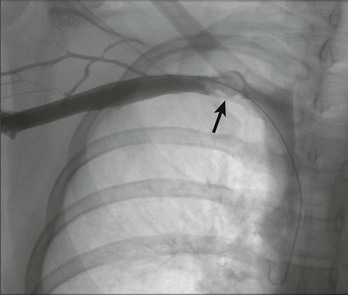
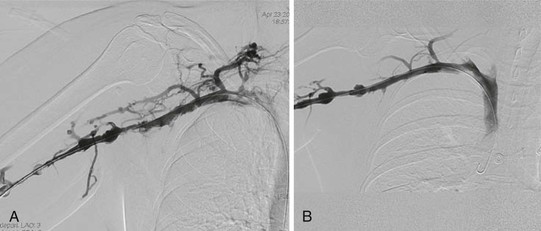
Venous duplex ultrasound is used as the initial diagnostic evaluation of patients who present with arm edema and pain. Whereas venography remains the “gold standard” for the diagnosis of venous TOS, it is generally reserved for patients whose condition warrants an intervention. The patient is placed in the supine position, and the entire arm is circumferentially prepped into the field. Under ultrasound guidance, the basilic vein is punctured percutaneously. Cephalic vein access will often result in an incomplete diagnostic study because the cephalic vein drains directly into the subclavian vein, thereby bypassing the axillary vein; thrombosis of this critical portion will therefore be missed. If thrombosis is visualized (Fig. 128-2), thrombolysis of the subclavian-axillary vein is initiated. Once thrombolysis is successful or in patients found to have a patent but severely narrowed subclavian-axillary vein, positional venography is performed. Positional venography of the subclavian-axillary vein segment is performed in full adduction and then in 90 degrees of abduction with external rotation (“hand-on-head” position) (Fig. 128-3). These positional images are essential to confirm the presence of extrinsic compression of the subclavian vein at the level of the thoracic outlet.
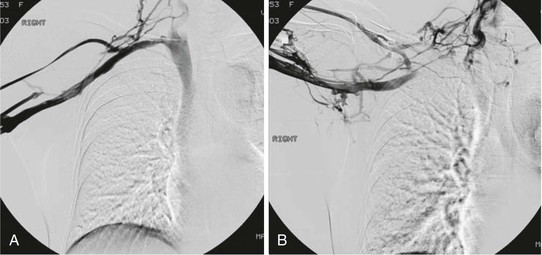
Figure 128-3 Venogram of the subclavian-axillary vein after successful thrombolysis with the extremity in full adduction (A) and in full abduction (B).
Evaluation of the collateral circulation is another important function of venography. The extent of collateralization provides information about the hemodynamic significance of the occlusion or stenosis and about the chronicity of the occlusion. Chronicity of the occlusion, in turn, plays a key role in dictating management and therefore has therapeutic implications. Accordingly, I classify patients into one of three categories on the basis of their history and venographic findings: (1) acute subclavian-axillary vein thrombosis, (2) chronic or recurrent subclavian-axillary vein thrombosis, or (3) high-grade symptomatic subclavian-axillary vein stenosis.
Treatment
Anticoagulation Alone
Historically, treatment of acute primary subclavian-axillary vein thrombosis consisted of rest and elevation of the affected extremity, accompanied by a variable duration of systemic anticoagulation. Several retrospective reviews have demonstrated this approach to venous TOS to be associated with significant long-term morbidity and patient disability.1,10,16,26,42 Hughes reported on the conservative management of 320 patients with primary subclavian-axillary vein thrombosis, 40% of whom had persistent symptoms or limited recovery.1 Likewise, in separate publications, Adams and DeWeese, Tilney and colleagues, and Urschel and Razzuk each reported high rates of residual functional impairment after treating patients with venous TOS in this manner (approximately 70%, 75%, and 74%, respectively).10,26,27
Thrombolytic Therapy
The early use of catheter-directed thrombolysis has emerged as the preferred initial management strategy in the modern treatment paradigm of venous TOS.12,27,38,43–47 Because subclavian-axillary thrombus is much more localized than lower extremity DVT, this approach usually lyses the subclavian-axillary vein clot quickly to restore luminal patency. In 1981, Zimmermann and associates documented, in a small case series, the successful use of systemic urokinase in 82% of patients with acute primary subclavian-axillary thrombosis.48 Since this initial series, many authors have demonstrated high rates of success in re-establishing patency with catheter-directed thrombolysis (Fig. 128-4).38,43–46 Lee and colleagues reported their experience treating 64 patients with primary subclavian-axillary thrombosis. As the first step in their treatment algorithm, 54 patients with symptom onset within 7 days underwent attempted catheter-directed thrombolysis; 100% experienced successful restoration of luminal patency.38 If thrombolysis is initiated within 14 days of the onset of symptoms, the results are generally reported to be excellent.9,44 Treatment with thrombolysis in patients with more than 14 days of symptoms is possible,43,46 albeit with a decreased chance for successful re-establishment of luminal patency. However, Guzzo and associates recommend against preoperative thrombolysis because long-term outcomes in their experience were no different from those achieved by preoperative anticoagulation alone.49 However, in the latter series, no objective determination of presence or absence of venous TOS as a cause of the subclavian vein thrombosis was established.
Thrombolysis Technique.
Although numerous thrombolytic agents and devices are available, the technical concepts for all are similar. Under ultrasound guidance, the ipsilateral basilic vein is accessed, and a wire is advanced centrally across the thrombosed vein into the superior vena cava (SVC) or right atrium. A catheter containing side holes for delivery of the thrombolytic agent is then positioned across the thrombosed region. An infusion of thrombolytic agent is then commenced and continued for usually less than 48 hours, with intermittent reimaging to assess for progress. See Chapter 36 for a complete review of the pharmacology related to the available thrombolytic agents.
Alternatively, numerous mechanical thrombectomy devices that feature clot maceration and aspiration can be used at the initial procedure and during subsequent reimaging.50–52 The two devices commonly used by the author’s group are the AngioJet (Possis, Minneapolis, Minn) and the Trellis Peripheral Infusion System (Bacchus Vascular, Santa Clara, Calif). These technologies, albeit at greater cost, allow more rapid clot dissolution than can be achieved with traditional thrombolysis infusion. The AngioJet functions by high-pressure injection of fluid through a distal catheter pore with simultaneous rapid aspiration through an adjacent pore. Secondary to the Bernoulli effect, an eddy current is created that results in low pressure and subsequent clot maceration and aspiration. In contrast, the Trellis system is a multilumen catheter with two compliant balloons at the distal end and infusion holes between these balloons. An integrated wire is connected to a drive unit that oscillates the wire within the isolated region to macerate the clot and to disperse the infused fluid (i.e., lytic agent). The isolated area between the occluding balloons can then be aspirated through the catheter lumen.
Post-Thrombolysis Management.
At the conclusion of thrombolysis, patients can be divided into two major categories: unsuccessful thrombolysis and successful thrombolysis. Patients with unsuccessful thrombolysis, defined as persistent total occlusion of the subclavian-axillary vein, should receive warfarin anticoagulation and measures aimed at control of local symptoms, such as rest, compression, and elevation. Unsuccessful thrombolysis of acute subclavian vein thrombosis is rare and almost always seen in patients who have had repeated episodes of lysis, anticoagulation, and rethrombosis. Although I do not offer these patients surgical treatment, a limited number of authors, in small series, have recommended aggressive regimens that include thoracic outlet decompression with variable lengths of chronic anticoagulation. Using this strategy in a group of 42 patients, Urschel and Razzuk reported a 57% rate of recanalization and resolution of symptoms.27 de León and colleagues reported 100% success in their small case series of four patients who underwent thoracic outlet decompression and were then maintained on chronic anticoagulation until recanalization was documented.53 In contrast to these recommendations for patients with acute subclavian vein occlusion, it is not surprising that Guzzo and colleagues found no benefit of endovascular intervention to restore patency before first rib resection in the management of 65 patients with chronic subclavian vein thrombosis.49
Patients in whom thrombolysis was successful, defined as those with re-establishment of subclavian-axillary vein patency, can be further subdivided, on the basis of completion positional venography, according to the absence or presence of demonstrable extrinsic compression at the thoracic outlet. Patients without extrinsic compression will not benefit from thoracic outlet decompression and should be treated with 3 to 6 months of systemic anticoagulation.54
In patients found to have venous TOS, no effort is made at the completion of successful thrombolysis to dilate a persistent subclavian vein stenosis. I favor immediate thoracic outlet decompression and intraoperative venography and subclavian vein angioplasty. At the other end of the spectrum, some recommend 3 to 6 months of systemic anticoagulation after successful thrombolysis.18,55 Management of patients with extrinsic compression, or radiographically documented venous TOS, is discussed next in surgical decompression of the thoracic outlet.
Surgical Decompression of the Thoracic Outlet
Patients with persistent stenosis or signs of extrinsic compression on positional venography after thrombolysis remain at significant risk of recurrent thrombosis with anticoagulation alone.46 In addition, if the underlying pathophysiologic process has not been addressed, adjunctive therapies such as balloon angioplasty or stent placement may provide satisfactory immediate results but lack sufficient durability to be used as definitive therapy.38,43,56–58 Multiple reports have confirmed that the radial force associated with either a self-expanding or balloon-expandable stent is not adequate to compensate for the compressive force between the first rib and clavicle; stent deformation, fracture, and thrombosis in this setting are the norm rather than the exception (Fig. 128-5). Therefore, stents have no role in the treatment of venous TOS before surgical decompression.
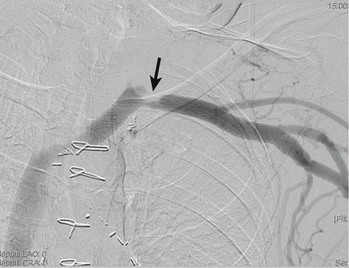
Figure 128-5 Deformed balloon-expandable stent 6 weeks after treatment of primary subclavian-axillary thrombosis.
Once subclavian-axillary vein patency has been restored and extrinsic compression has been demonstrated, first rib resection with external venolysis should be performed. Although some authors advocate deferring surgical decompression for 1 to 3 months after thrombolysis to allow healing of the venous endothelium and resolution of the acute inflammatory process,18,55 most now agree that decompression should take place during the same hospitalization as the thrombolysis to decrease the significant risk of reocclusion that may occur between thrombolysis and deferred surgery.9,27,43,45,46,59,60 Immediate operative decompression, even as early as 4 hours after thrombolysis,61 has been demonstrated to be safe and effective. This shift to immediate surgery has virtually eliminated the vein rethrombosis rate of 6% to 18% that has been reported to occur during the waiting period.9,55,62 Nonetheless, should the patient experience rethrombosis while awaiting surgical decompression, thrombolysis can be reinitiated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree