IMAGING AND OTHER DIAGNOSTIC STUDIES
■ Transesophageal echocardiography (TEE) may serve as a useful imaging tool, particularly in the setting of acute thoracic aortic pathology. TEE can confirm the presence of aortic dissection, distinguish between types A and B dissections, identify involvement of supra-aortic vessels, and assess for contained rupture.
■ High-resolution computed tomography angiography (CT-A) with three-dimensional reconstructive software allows for the most complete anatomic analysis, including details regarding aneurysm morphology, diameter, dissection flap characterization, thrombus burden, calcification, angulation, and branch vessel orientation.
■ Familiarity and routine usage of three-dimensional workstations and the ability to customize measurements provide an accurate road map to guide endovascular strategy, device selection, and stent graft sizing.
SURGICAL MANAGEMENT
Preoperative Planning
■ Patients scheduled for elective TEVAR undergo routine preoperative cardiac evaluation. Based on cardiovascular risk profile, symptomatology, and presence of electrocardiogram abnormalities, selected patients undergo further evaluation in the form of an exercise stress test, dobutamine stress echocardiography, or Persantine thallium stress testing. Coronary angiography is pursued in cases involving extensive or symptomatic coronary artery disease.
■ Aortic transections or symptomatic dissections and aneurysms should have early and aggressive blood pressure control using intravenous beta-blocker or calcium channel blocker medications. After obtaining a reliable clinical examination, refractory chest, back, or abdominal pain should be treated with narcotic analgesics.
■ Renal protective strategies should be employed preoperatively to minimize the risk of contrast-induced nephropathy. Intravenous hydration is initiated preoperatively and, in the setting of baseline renal insufficiency, may warrant early hospital preadmission and concomitant administration of Mucomyst and bicarbonate infusion.
■ Suspected blunt aortic injury should prompt a referral to a level I trauma center in order to facilitate early evaluation by a vascular specialist and other pertinent members of a multidisciplinary trauma team.
■ General anesthesia is routinely performed in TEVAR cases. Prophylactic lumbar cerebrospinal fluid (CSF) drainage is considered in every case based on the relative risk of spinal cord ischemia, hemodynamic status, and acuity of clinical presentation. Arterial monitoring is performed via a right radial artery approach. Peripheral intravenous lines are typically adequate; however, more intensive central venous monitoring may be required in cases involving unstable traumatic transections, patients with significant baseline cardiovascular comorbidities, or any case involving hemodynamic instability.
■ Preoperative imaging should be heavily scrutinized for the adequacy of iliofemoral access anatomy. An iliac conduit may be required in cases involving small-caliber, tortuous, or heavily calcified access vessels. Anticipated use of a conduit should prompt consideration of an autotransfusion or cell saver machine to be available during the procedure.
■ Numerous variables have been identified as risk factors for the development of spinal cord ischemia after TEVAR. Given that hypoperfusion represents the primary etiology of spinal cord injury following TEVAR, commonly cited risk factors involve those relating to the extent of impairment or exclusion of the collateral perfusion to the spinal cord. The European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) investigators reported results from the largest multicenter registry to date (N = 606).5 In the EUROSTAR registry, the incidence of spinal cord ischemia was 2.5% and independent risk factors included left subclavian artery coverage without revascularization (odds ratio [OR], 3.9; p = .037), concomitant open abdominal aortic surgery (OR, 5.5; p = .037), and the use of three or more stent grafts (OR, 3.5; p = .043).
■ Based on the principle that spinal cord perfusion pressure is approximated by the difference between the mean arterial pressure (MAP) and CSF pressure, placement of a prophylactic lumbar drain has the potential to increase spinal cord perfusion pressure by decreasing CSF pressure and may be beneficial in select patients at high risk for spinal cord ischemia. Percutaneous drainage of CSF is performed by inserting a silastic catheter 10 to 15 cm into the subarachnoid space through a 14-gauge Tuohy needle at the L3–L4 vertebral interspace. The open end of the catheter is attached to a sterile closed circuit reservoir and the lumbar CSF pressure is measured with a pressure transducer zero-referenced to the midline of the brain. Lumbar CSF can be drained continuously or intermittently in the operating room to achieve target CSF pressures of 10 to 12 mmHg. Postoperatively, intermittent or continuous CSF drainage can be continued in the intensive care unit for CSF pressures exceeding 10 mmHg or at the first sign of lower extremity weakness. In the absence of neurologic deficits, the lumbar CSF drainage catheter can be clamped 24 hours postprocedure followed by continued monitoring of CSF pressure together with serial neurologic assessments. The CSF drain can then be removed at 48 hours after operation. Although prophylactic or therapeutic lumbar CSF drainage has an established record of safety, complications have been reported to occur in approximately 1% of patients, which may include neuraxial hematoma, subdural hematoma, catheter fracture, meningitis, intracranial hypotension, chronic CSF leak, and spinal headache.
Selection and Sizing of Thoracic Stent Graft
Landing zones
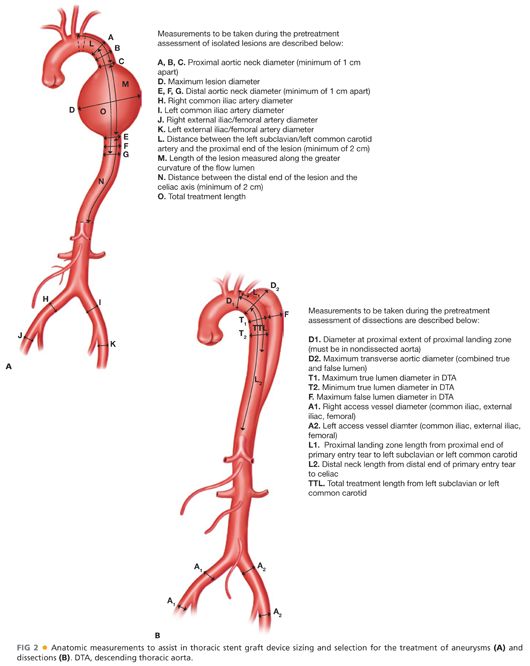
■ Proximal and distal landing zones must be of sufficient length (usually at least 2 cm) to enable safe and accurate deployment bracketing the area of thoracic aortic pathology, which often includes the subclavian artery proximally or the celiac artery distally.
■ Intentional coverage of the left subclavian artery is sometimes required due to a very proximal extent of aortic pathology, especially transections. Left subclavian artery revascularization may be required in select cases. The celiac artery rarely requires intentional coverage.
■ Significant tortuosity, circumferential mural thrombus, and extensive calcification can compromise the proximal or distal landing zone, thereby predisposing to inadequate fixation and subsequent development of endoleak or migration. Site of proximal and distal landing zones should be selected in order to minimize the impact of these anatomic features, even if it requires extending the length of aortic coverage.
■ A variety of anatomic measurements are taken from preoperative CT-A imaging to assist in the sizing and selection of the thoracic stent graft (FIG 2). Interventionalists should be proficient in accurate sizing and measuring of key thoracic aortic locations that influence device selection and ultimately determine patient outcomes.
Sizing of stent grafts
■ The degree of stent graft oversizing can vary based on the indication for intervention. Stent grafts are generally oversized by 10% to 20% based on the aortic diameter at the proximal and distal fixation sites for aneurysmal disease. Insufficient oversizing for the treatment of TAAs may predispose to inadequate exclusion and the potential for endoleak or migration. Aggressive oversizing, on the other hand, increases the risk for stent graft collapse, graft thrombosis, access arterial injury, and potential for peri- or postprocedural iatrogenic retrograde type A dissection.
■ Chronic type B dissections are frequently characterized by a thick, nonmobile dissection flap, or septum, that separates true and false lumens into concave or convex discs of flow lumen. Such dissection flaps have limited compliance; therefore, minimal or no oversizing may be required in order to achieve a suitable proximal or distal seal.
■ Aortic transections frequently occur in young trauma patients with normal or minimally diseased aortas. As such, minimal oversizing is needed to achieve an adequate seal and only recently did device manufacturers create devices meant for smaller diameter aortas. Note also that under-rescucitated patients on admission will have smaller aortic diameters on their CT-A.
■ Currently available stent grafts range in diameter from 22 to 46 mm. Given the traditional 10% to 20% rule of device oversizing, these devices are designed to safely treat aortas with landing zones ranging from 19 to 43 mm in diameter.
Access vessel anatomy
■ Current thoracic aortic stent grafts require large-caliber delivery systems, ranging from 18 to 26 Fr in outer diameter. Small, tortuous, and heavily calcified iliofemoral arteries may prohibit sheath advancement and predispose to access site–related complications, including groin hematoma, dissection, or rupture.
■ Careful evaluation of access vessel anatomy on preoperative imaging should be performed in order to assess the caliber, tortuosity, thrombus burden, and extent of calcification of the iliofemoral arteries. Such anatomic information will serve as the basis for deciding laterality of femoral access as well as to determine the need for an iliac conduit.
■ Serial dilation may be attempted for patients with small iliofemoral vessels. Iliac atherosclerotic lesions may be pretreated with balloon angioplasty and/or stent grafting in order to facilitate sheath advancement and introduction of the thoracic stent graft components.
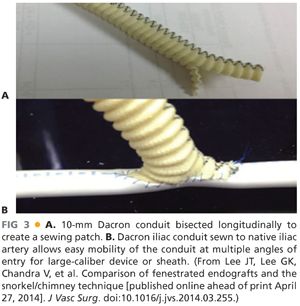
■ Iliac conduits serve as a safe and reliable technique to circumvent issues related to suboptimal access vessel anatomy. From either flank incision, a retroperitoneal exposure provides visualization of the common iliac artery or distal abdominal aorta. A 10- or 12-mm Dacron graft is commonly used as the conduit of choice. The conduit can be modified by creating a patch at the distal end in order to further facilitate the delivery of large-caliber sheath and enable additional degrees of torqueability (FIG 3). This modification involves creating a patch by cutting the Dacron graft along its long access, thereby enlarging the transition zone from the graft to artery.
TECHNIQUES
EARLY PROCEDURAL CONSIDERATIONS
Positioning
■ The C-arm is typically configured in the “head” position. The left arm may be abducted to 75 to 90 degrees and circumferentially prepped into the field if an embolization or snorkel/chimney procedure involving the left subclavian artery is anticipated. The chest, abdomen, and bilateral groins should be prepped. As frequently only one groin access is required for the performance of a routine TEVAR, laterality of the operator position may vary based on surgeon preference or anticipated access site location.
Establishing Vascular Access
■ The ipsilateral femoral artery is accessed either percutaneously or from an open exposure. Secondary access may be obtained from the contralateral femoral artery or brachial artery as needed for a 5-Fr sheath and flush catheter. Surgical exposure is obtained from a small oblique incision at the level of the inguinal ligament. The common femoral artery is exposed, with proximal control obtained at the level of the external iliac artery and distal control at the level of the femoral bifurcation or proximal superficial femoral and profunda femoral arteries. Heavy calcification may require preemptive endarterectomy and patch angioplasty in order to facilitate safe sheath placement.
■ The femoral artery is punctured using a standard micropuncture set, and if arterial access is obtained percutaneously, a sheathogram is performed to confirm adequate puncture site location (mid–common femoral artery). A standard length Bentson wire is inserted into the aorta through micropuncture sheath and exchange for a 7-Fr sheath is then performed using Seldinger technique. Wire exchange is then done for a 260-cm stiff Lunderquist wire. The Lunderquist wire should have a flexible, curved proximal end that should be advanced under fluoroscopy across the aortic arch to abut the aortic value. The location of the distal end of the Lunderquist wire should be marked on the operating table and this wire position should be maintained throughout the procedure.
■ Over the stiff Lunderquist wire platform, the 7-Fr sheath is removed and serial dilators are advanced to gradually enlarge the subcutaneous tract and arteriotomy site in order to accommodate either the stent graft device itself or a larger 18- to 26-Fr introducer sheath required for device delivery.
■ After placement of the larger sheath, systemic heparin is administered at a dose of 100 units/kg (goal activated clotting time of >250 seconds). Concomitant traumatic injuries, particularly intracranial hemorrhage, may alter the dose or decision to administer heparin.
INITIAL AORTOGRAM
■ A 5-Fr 100-cm Omniflush or pigtail catheter is inserted into aorta and advanced to the level of the aortic arch. This catheter may be advanced via a contralateral 5-Fr sheath or it may be inserted into an additional ipsilateral 5-Fr sheath placed distal to the arteriotomy for the main body delivery sheath.
■ If satisfied with stent graft sizing based on available preoperative imaging, the thoracic aortic stent graft may be advanced over the Lunderquist wire and be positioned in the proximal to midportion of the thoracic aorta prior to initial aortogram.
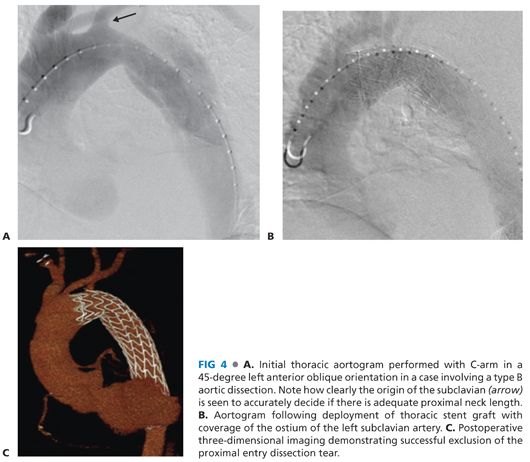
■ Optimal angiographic imaging of the aortic arch is obtained by placing the fluoroscopic C-arm in a left anterior oblique orientation, often 35 to 65 degrees, and can be optimized by referencing the preoperative CT-A. The location of the supra-aortic vessels, particularly the left subclavian artery, should be noted and marked on viewing monitors (FIG 4A).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


