Fig. 13.1
Lateral view of the great vessels of the aorta
13.2.3 Descending Aorta
The descending aorta begins after the ligamentum arteriosum and travels to the aortic hiatus at the level of the diaphragm near the T12 vertebra, where it becomes the abdominal aorta. The thoracoabdominal aorta lies posterior to the esophagus at this level, near which it gives off bronchial arteries, esophageal arteries that travel to the middle third of the esophagus, several mediastinal arteries that travel to the posterior mediastinum, pericardial arteries, nine pairs of posterior intercostal arteries that supply the 3rd–11th intercostal spaces, two subcostal arteries, superior phrenic arteries that supply the diaphragm, and a number of lumbar branches that travel to the spinal cord.
13.2.4 Variant Anatomy
A variety of congenital alterations to the aortic arch exist, such as the true bovine arch and so-called bovine arch. The true bovine arch is a rare variant in which a single, large common brachiocephalic trunk comes off the arch and separates into the right and LSAs and the bicarotid trunk. This bicarotid trunk then bifurcates to form the right and left common carotid arteries. A more common variant is the so-called bovine arch, present in as many as 20 % of patients [4]. The so-called bovine arch includes a brachiocephalic trunk that splits into the right subclavian, right common carotid, and left common carotid arteries. The LSA comes off separately from the arch of the aorta. Other variants include the left vertebral artery coming off directly from the arch in 2.5 % of patients and the existence of a right-sided aortic arch in patients with dextrocardia or situs inversus.
Anatomic variants with the aorta can masquerade as traumatic injury. For example, a ductus diverticulum may be present along the anterior surface near the ligamentum arteriosum, which may be confused with pseudoaneurysm formation. Ductus diverticula appear as a smooth outline within the aortic lumen and do not have intimal flaps. In contrast, true pseudoaneurysm formation typically presents as an irregular outpouching with an irregular base and intimal flaps. An aortic spindle may present as circumferential dilatation between the LSA and ligamentum arteriosum.
13.3 Pathophysiology
More than 85 % of patients with blunt TAI die prior to hospital arrival. The majority of patients who survive to undergo imaging and intervention have an injury at the aortic isthmus that is limited to the intima and media [5]. Survival of more advanced injuries (free rupture of the aorta) is rare [6, 7]. Nearly 99 % of patients who survive to make it to the hospital will die without surgical intervention: 15 % will die within the first hour, 30 % within 6 hours, nearly 50 % within 1 day, nearly 75 % within 1 week, and 90 % within 4 months [5]. The most common site of blunt TAI is at the proximal descending aorta, near the location of the ligamentum arteriosum, occurring at a rate of approximately 46 %. Elsewhere within the thoracic aorta, the rate of injury is 18 % at the ascending aorta, 13 % at the arch, and 16 % at the descending aorta (Fig. 13.2) [5, 8–12].
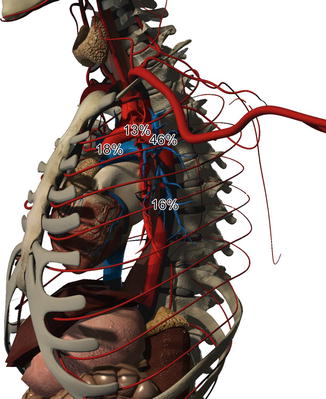

Fig. 13.2
The incidence of injury to the thoracic aorta ranges from 18 % at the ascending aorta, 13 % at the arch, 46 % at the ligamentum arteriosum, to 16 % at the descending aorta
13.3.1 Mechanism of Injury
The cause of blunt TAI is multifactorial and includes high-shearing forces, torsion, and extreme stretch at fixed points of the aorta, such as the ligamentum arteriosum, aortic root, and sites of major branches, such as the arch [7]. Differential relative motion at different points of fixation further contributes to trauma, especially at the isthmus [13, 14]. Penetrating injuries to the thoracic aorta are uncommon. When present, they are likely to lead to rapid exsanguination, and many of these patients do not survive transport to the hospital. For those rare survivors, open repair is indicated for unstable patients. Endovascular repair may be attempted in a stable patient who is an anatomically suitable candidate based on preoperative imaging.
13.3.2 Classification of Injury
Based on CTA imaging, TAI is classified by severity: grade 1 injuries have an intimal tear (without external contour abnormality); grade 2 injuries involve the media and develop an intramural hematoma (with external contour abnormality); grade 3 extend to the adventitia and lead to pseudoaneurysm formation; and grade 4 have a free rupture of the aorta (Figs. 13.3 and 13.4) [15]. Management of grade 1 injuries can be conservative, with strict blood pressure control and follow-up CTA in 6 weeks to verify resolution. Grade 2 and 3 injuries should be urgently repaired, while grade 4 injuries require emergent intervention.
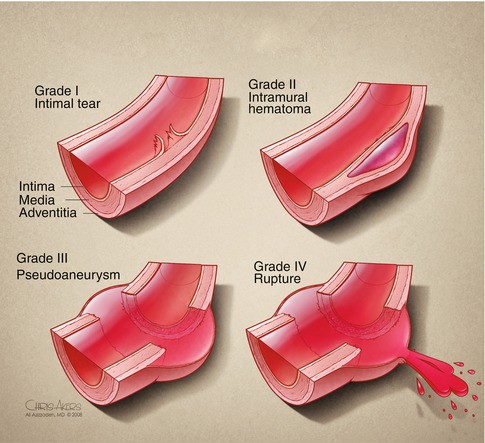
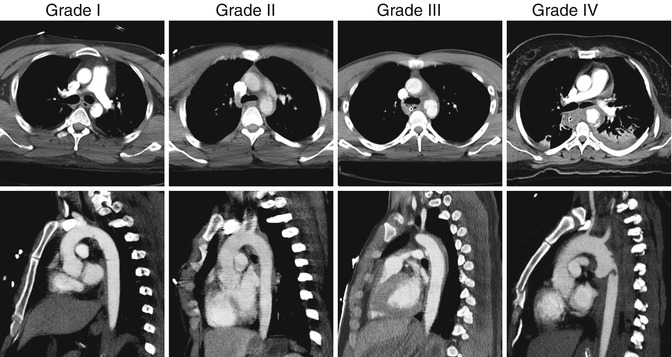

Fig. 13.3
Classification of traumatic aortic injury. Grade I injuries present with an intimal tear and can often be managed with anti-impulse control. Grade II injuries have an intramural hematoma and are often managed with endovascular stent graft placement. Grade III injuries develop a pseudoaneurysm and should be covered with a stent graft to avoid rupture. Grade IV injuries have frank rupture of the aorta and require emergency surgery

Fig. 13.4
CTA images of patients with grade I–IV traumatic aortic injuries
13.4 Clinical Presentation
The most common causes of blunt TAI include motor vehicle collision (MVC) (78 %), motorcycle accident (9 %), pedestrian (7 %), fall (5 %), and bicycle (1 %) [9]. The use of a seat belt decreases the risk of TAI by fourfold [16]. Concurrent injuries in patients with blunt TAI include major abdominal injury (57 %), closed head injury (50 %), major peripheral vascular injury (46 %), multiple rib fractures (46 %), pulmonary contusion (38 %), pelvic trauma (31 %), upper extremity trauma (20 %), flail chest (12 %), and spine injury (12 %) [8, 17].
Up to one-third of patients with blunt TAI have minimal signs of chest wall injury, which may contribute to missed injuries in favor of more obvious orthopedic or peripheral vascular trauma. Physical exam findings are nonspecific and are not sensitive for the diagnosis of blunt TAI (Table 13.1). Penetrating TAI typically presents with evidence of chest wall trauma, and physical findings include distended neck veins, muffled heart sounds, and hypotension secondary to tamponade physiology.
Table 13.1
Stigmata of aortic injury on clinical examination
Symptoms | Signs |
|---|---|
Chest paina | Unexplained hypotension |
Dyspnea | Upper limb hypertensionb |
Cough | Decreased lower limb pulsesc |
Dysphagia | Systolic murmurd |
Hoarsenesse | Anterior chest wall contusion |
Hemoptysisf | Sternal or thoracic spine fractures |
Expanding hematoma at the thoracic outlet |
13.5 Diagnosis
The diagnosis of TAI in patients with blunt trauma can be suspected on plain chest x-ray (Table 13.2). A widened mediastinum may be seen on plain chest x-ray in more than 90 % of patients with TAI [18, 19]. CTA is highly sensitive (95–100 %) with a negative predictive value approaching 100 % for TAI [18, 20–22]. False-positive studies, however, are possible [23, 24]. While CTA is the diagnostic modality of choice, patients with equivocal studies can undergo traditional angiogram and intravascular ultrasound (IVUS). If additional imaging is required after an equivocal CTA, we have found that IVUS is more sensitive than angiography. Therefore, we advocate the use of IVUS in suspected TAI patients for whom angiography is being considered (Fig. 13.5) [25].
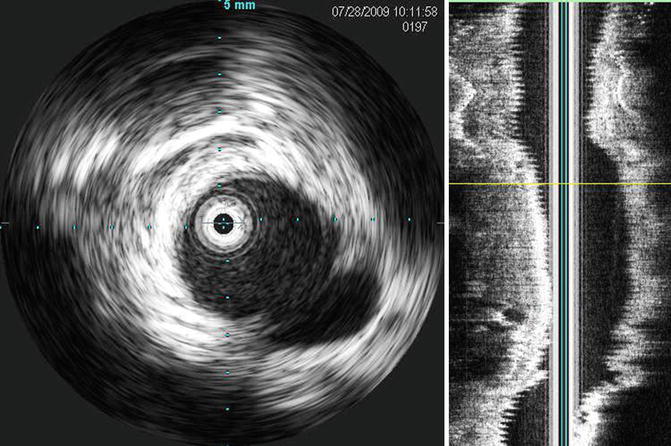
Table 13.2
Stigmata of aortic injury on plain chest radiography
Widened mediastinum |
Abnormal aortic outline |
Loss of the aortopulmonary window |
Depression of the left mainstem bronchus (more than 140° from trachea) |
Lateral deviation of trachea to the right of midline |
Lateral deviation of nasogastric tube to the right of midline |
Widened right paratracheal stripe |
First and second rib fractures |
Sternal fracture |
Multiple rib fractures with a crushed chest |
Large hemothorax |
Left apical cap (hematoma) |
Clavicular fracturea |
Anterior displacement of trachea (lateral radiograph) |

Fig. 13.5
Intravascular ultrasound in a patient with a grade III traumatic aortic injury. The pseudoaneurysm can be seen in the 4 o’clock position
13.6 Management
The initial management of patients with TAI should be to initiate the advanced trauma life support (ATLS) guidelines, including an assessment of the airway, breathing, circulation, disability, and exposure. Immediate management of life-threatening injuries should be the focus. Permissive hypotension should be the norm to avoid exacerbating major vascular injuries, and elevations in blood pressure should be avoided to minimize the risk of vascular rupture. Specifically, a mean arterial pressure below 80 mmHg should be the target [26]. There should be an appropriate balance between permissive hypotension and maintaining a satisfactory cerebral perfusion pressure. The patient’s overall hemodynamic status dictates the management strategy. Hemodynamically unstable patients should be managed in the operating room, especially those with major concurrent trauma. Patients with high-volume chest tube drainage (i.e., more than 1,500 mL or a rate greater than 250 mL/h for 4 h) should also be managed in the operating room [27].
13.6.1 Medical Management
Patients with grade I TAI can be treated medically. Decreasing the intra-aortic shear force by decreasing blood pressure can be achieved using beta-blockers and other antihypertensive agents. Use of a nicardipine and esmolol drip should be initiated in patients who do not promptly respond to oral or intermittent intravenous therapy to decrease their heart rate below 100 beats per minute and blood pressure below 100 mmHg. Replacement of the drips with an oral regimen should be instituted over a period of days. New symptoms, continuing pain, or progression of injury on a CT scan are indications for operative intervention [28].
13.6.2 Delayed Surgical Management
Delayed repair (>24 h) may be suitable for selected patients with grade 2 or 3 injuries who have concurrent severe trauma [29, 30]. For example, patients with severe closed head injuries, severe sepsis, or major multisystem trauma may require more immediate management of life-threatening injuries prior to treatment of TAI. Strict blood pressure control is necessary, and some delay in operative management may improve physiologic optimization without compromising overall care [27, 31].
13.6.3 Operative Management
13.6.3.1 Open Repair
Open repair commences following general anesthesia via a double-lumen endotracheal tube. Aortic perfusion distal to the arch is achieved using a Bio-Medicus (Minneapolis, MN) pump with an inline heat exchanger. This is used with outflow cannulation from the left inferior pulmonary vein to either the distal descending aorta or left common femoral artery. An alternative strategy includes cannulating the left atrium or superior pulmonary vein, depending on what structure is most accessible. The patient is therapeutically heparinized and the aorta cross-clamped proximal to the LSA in cases of TAI to the isthmus. An alternative approach to therapeutic heparinization is to use a Carmeda®-coated circuit, in which only 5,000 units of heparin need to be administered. A longitudinal incision is made to visualize the tear, and hemostasis achieved. Transverse incisions can also be utilized to divide the aorta, which permits inspection and debridement of the ends of the aorta. The site of injury is bypassed using a woven Dacron tube graft, which is sewn in place with a running 4–0 pledgeted polypropylene suture at both the proximal and distal anastomosis. Protamine is given to reverse the heparin and the patient transported to the intensive care unit (ICU) [15].
13.6.3.2 Endovascular Repair
Following induction of general anesthesia, endovascular repair is performed in an operating room equipped with imaging technology. The abdomen and bilateral groins are prepped. A diagnostic arch aortogram is performed through percutaneous femoral artery access. The cerebrovascular anatomy is evaluated, especially if LSA coverage is required. The patient is anticoagulated with intravenous heparin, but at a lower dose (0.5 mg/kg) than the weight-based protocol. A thoracic stent graft is selected according to the manufacturer’s instructions for use and based on cross-sectional measurements of the aorta. The device is delivered via open or percutaneous femoral artery access. Once deployed, a completion aortogram is performed to ensure satisfactory placement and ensure that there are no endoleaks (Table 13.3). Selective balloon angioplasty can be performed as necessary if there is concern about graft apposition. The heparin is reversed with protamine. The patient is then transferred to the trauma ICU.
Table 13.3
Types of endoleaks
I. Incomplete seal at either side of the stent graft |
II. Blood accumulation in the excluded segment as a result of back bleeding from branches covered by the stent graft
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|