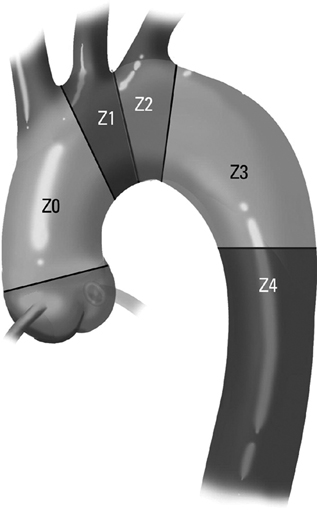
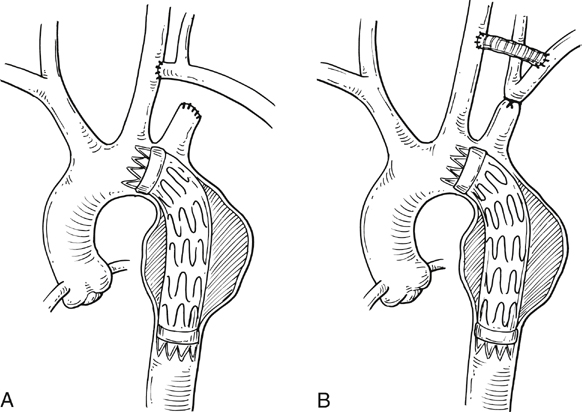
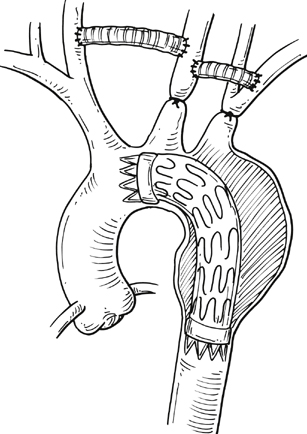
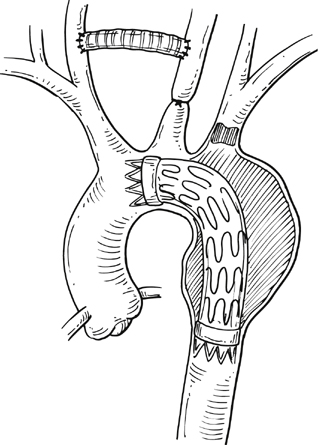
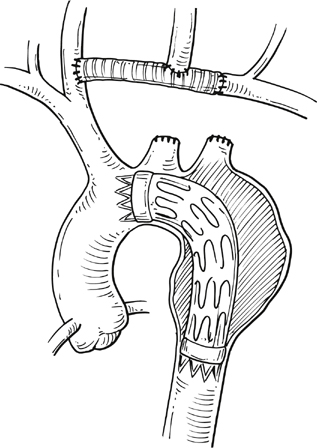
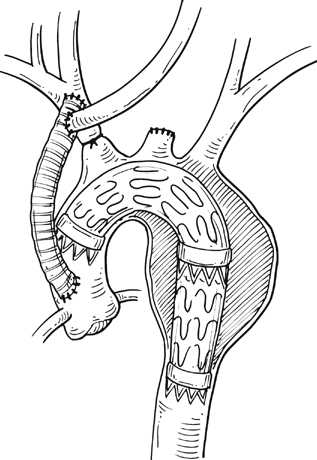
Commercially available thoracic aortic stent grafts are typically deployed distal to the origin of the left subclavian artery (SCA), with a 2- to 2.5-cm requirement for the proximal landing zone to achieve a proximal seal (Figure 1). Landing zone 0 involves the ascending aorta proximal to the innominate artery, zone 1 involves the aortic arch between the innominate and left common carotid artery (CCA), zone 2 involves the aortic arch between the left CCA and the left SCA, zone 3 involves the proximal descending thoracic aorta distal to the left SCA, and zone 4 involves the mid descending thoracic aorta. For zone 2 TEVAR implantations, left SCA revascularization can be accomplished either with transposition of the left SCA and the left CCA (Figure 2A) or with left CCA to left SCA bypass, usually with 8-mm ringed polytetrafluoroethylene (PTFE) graft (Figure 2B). Potential differences with respect to patency and infection rates between these two interventions have never been definitively established. Initially, the left SCA was considered somewhat superfluous (in the absence of internal mammary–based coronary circulation or dominant vertebral flow), and coverage with the aortic endograft proximally was routinely performed with little perceived risk. Recent research has demonstrated a greater clinical importance of this vessel. When the left SCA is intentionally occluded proximally in the absence of revascularization, vertebral artery blood flow becomes retrograde and provides inflow for the arm. By providing antegrade flow to the left SCA from the carotid, antegrade left vertebral and internal mammary flow is maintained. Controversy still exists regarding routine revascularization and selective revascularization of the left SCA when endografting covers the origin of this artery. In situations where zone 1 implantation is intended, a left CCA bypass is needed. This can be combined with revascularization of the left SCA as previously described. Typically the left CCA is revascularized through a carotid-to-carotid bypass graft (Figure 3). Tunneling can be accomplished through either a retropharyngeal or subcutaneous route. It has been our preference for the former to avoid potential complications with a median sternotomy if one might be needed in the future. Concomitant left SCA bypass can be performed depending upon the preoperative workup to ensure adequate collateral circulation, especially in the vertebral artery territory. The proximal left SCA in nearly all situations is either embolized through percutaneous techniques or ligated to prevent a type II endoleak (Figure 4). Other techniques have been described and include right CCA to left SCA bypass with implantation of the left CCA to the bypass (Figure 5). For zone 0 treatments, the patient requires a median sternotomy with ascending aortic side clamping. The bypass graft is constructed from the proximal ascending aorta to the innominate and left CCA arteries. The left SCA is often difficult to revascularize through the median sternotomy incision; the left SCA is typically revascularized through previously described techniques and before median sternotomy. Other methods exist to bypass the innominate and the left CCA including a single, large, 12- to 14-mm Dacron tube graft from ascending aorta to the innominate artery, followed by left CCA transposition onto the graft (Figure 6) or with a jump graft to the left CCA. Anastomotic configurations are typically end to end, with oversewing of the main stump. Trifurcated grafts or new hybrid grafts are available with side arms for reconstructing great vessels. An additional side arm is often incorporated that can be used to deploy the endograft in antegrade fashion without the need to access the femoral arteries (Figures 7 and 8).
Thoracic and Abdominal Aortic Debranching in the Endovascular Treatment of Thoracoabdominal Aortic Aneurysms
Aortic Arch and Proximal Descending Aortic Aneurysm Debranching
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
