Therapeutic Cardiac Catheterization
Ralf J. Holzer
John P. Cheatham
Introduction
Catheter-based techniques, whether palliative or corrective, are the accepted therapy for many congenital cardiac defects. Interventional, or, better termed, therapeutic catheterizations, were initiated by Dotter and Judkins, who first reported the treatment of peripheral vascular lesions during a catheterization in 1964 (1), when they dilated a stenotic peripheral vessel through a cutdown on the vessel. The next major innovative accomplishment and the first intracardiac therapeutic catheterization procedure for pediatric congenital heart disease were the balloon atrial septostomy done by Rashkind and Miller in 1966 (2). That procedure really “set the stage” for all therapeutic catheterization procedures used today. In 1967, Porstmann and colleagues reported the first nonsurgical corrective procedure in the catheterization laboratory with their description of a technique for closure of a patent ductus (3). Vascular occlusion coils were introduced by Gianturco and colleagues in 1975 (4) and in 1976 King and Mills were the first to describe the closure of atrial septal defects (ASDs) in the catheterization laboratory (5). Even though their device has not found widespread use, it set the stage for future development of transcatheter devices. One of the largest contributions to interventional cardiology has probably been made by Gruentzig, a Swiss-native who in 1976 reported on dilation of peripheral vessels with noncompliant balloons. This initiated a rapid innovative spurt within the congenital cardiac community during which narrowed lesions at various locations were treated with balloon angioplasty, frequently initially in a noncontrolled fashion. In 1982, Dr. Jean Kan reported the first successful transcatheter static balloon pulmonary valvuloplasty (6) and Dr. James Lock in 1987 used the clamshell device to occlude a ventricular septal defect (VSD) using a percutaneous approach (7). Dr. Charles Mullins introduced endovascular stents into the management of patients with congenital cardiac lesions (8), and the long list of innovations reached another milestone when Dr. Phillip Bonhoeffer, a German cardiologist working in France in 2000, performed the first transcatheter pulmonary valve replacement in a human (9). Transcatheter valve therapies and other interventional therapies to treat patients with structural heart disease have rapidly increased over the last few years. Another milestone was reached when the Melody valve (Medtronic, Minneapolis, MN) gained FDA HDE approval in January 2010. These therapies are not limited anymore to patients with congenital heart disease. In fact, transcatheter aortic valve implantations (TAVIs) in adults have overtaken transcatheter valve therapies in the congenital population, with thousands of implantation of the CoreValve (Medtronic, Minneapolis, MN) in adults having been performed thus far.
In this section, the most important therapeutic catheterization procedures performed as of this writing are discussed. This chapter is not intended as a complete and exhaustive textbook of interventional techniques, but instead should give the reader a general overview of therapeutic catheterization. Indications for the individual transcatheter interventions were recently published as a collaborative expert consensus statement from the AHA by Feltes and colleagues, and we have not repeated the entire article in this chapter, but strongly recommend to be aware of these recommendations (10).
Acknowledgment
We have used and expanded upon this chapter published in other editions of this textbook and therefore acknowledge the previous contributions made by Drs. Nancy Bridges, Martin O’Laughlin, Charles Mullins and Michael Freed.
It should be emphasized that not every pediatric cardiologist, or, for that matter, every center, should offer every therapeutic catheterization procedure. For any procedures to be performed at any particular institution, minimal specific skills are required, special techniques must be mastered and maintained, and a large inventory of specialized and expensive catheters and devices must be stocked to offer the patient an optimal procedure. Absence of appropriate qualifications and equipment can result in unnecessary risk to the patient without a reasonable chance of the therapeutic catheterization procedure being successfully accomplished. In fact, even if the patient is not acutely harmed by the attempt, it is important to be aware of the fact that the next procedure in a more appropriate setting might be compromised by a previously unsuccessful attempt.
Adverse Events and Quality Improvement
For many years, reporting of procedure-related adverse events was limited mostly to single-center retrospective experiences, often without any clearly and consistently applied criteria of what would be considered an adverse event, and how its severity should be defined (11,12,13). Over the years, several multicenter registries have captured procedural outcomes including efficacy and occurrence of adverse events, which included the VACA registry, MAGIC, as well as CCISC (14,15,16). The data derived from these registries often provided the only prospective multicenter outcome data for many procedure types.
A more systematic capture of procedure-related adverse events was accomplished recently through the C3PO multi-institutional registry (17), using definitions for event severity and preventability as defined in the International Pediatric Congenital Cardiology Code nomenclature (18). This registry documented not insignificant rates of adverse events, 10% for diagnostic cases, and 20% for interventional procedures. Higher severity (level 3 to 5) adverse events occurred in 9% of interventional cases, and 5% of diagnostic cases. The incidence of life-threatening adverse events has been reported to be as high as 2.1% (19).
However, to accurately compare adverse event rates and outcome between institutions and operators, an adjustment for case mix and hemodynamic vulnerability is required. Using consensus-based and empirical methods, and derived from the C3PO dataset, Bergersen and colleagues recently reported on procedure-type risk groups, separating different types of diagnostic and interventional procedures into four different risk groups, ranging from diagnostic procedures performed in patients over 1 year of age (Risk Group 1) to balloon angioplasty of four or more pulmonary artery branches (Risk Group 4) (20).
Following the definition of procedure-type risk groups, Bergersen and colleagues reported on hemodynamic variables associated
with vulnerability for AE, which were determined based on an empirical analysis of the C3PO data set. The combination of variables that were identified to offer the best predictive performance for high severity adverse events included systolic main PA pressure ≥45 mm Hg (two-ventricle) or mean PA pressure ≥17 mm Hg (single ventricle), systemic ventricular end-diastolic pressure ≥18 mm Hg, mixed venous saturations <60% (two-ventricle) or mixed venous saturation <50% (single ventricle), and systemic saturations <95% (two-ventricle) or <78% (single ventricle) (21,22). The CHARM model incorporates the procedure-type risk group, hemodynamic vulnerability, as well as age below 1 year as criteria to calculate a standardized adverse event ratio to facilitate an equitable comparison of adverse events between centers and operators (21). In addition, further work originating from the C3PO registry has found a weight of less than 2 kg as an additional independent risk factor for adverse events (23).
with vulnerability for AE, which were determined based on an empirical analysis of the C3PO data set. The combination of variables that were identified to offer the best predictive performance for high severity adverse events included systolic main PA pressure ≥45 mm Hg (two-ventricle) or mean PA pressure ≥17 mm Hg (single ventricle), systemic ventricular end-diastolic pressure ≥18 mm Hg, mixed venous saturations <60% (two-ventricle) or mixed venous saturation <50% (single ventricle), and systemic saturations <95% (two-ventricle) or <78% (single ventricle) (21,22). The CHARM model incorporates the procedure-type risk group, hemodynamic vulnerability, as well as age below 1 year as criteria to calculate a standardized adverse event ratio to facilitate an equitable comparison of adverse events between centers and operators (21). In addition, further work originating from the C3PO registry has found a weight of less than 2 kg as an additional independent risk factor for adverse events (23).
However, incidence of adverse events and efficacy of an interventional procedure don’t exclusively depend on patient-specific characteristics and procedure type. In fact, using data from the C3PO registry, Holzer and colleagues found that operators with fewer years in practice had higher risk-adjusted rates of adverse events (22,24,25).
These various quality improvement efforts have culminated in the development of IMPACT Registry, which is an initiative of the American College of Cardiology with support from The Society for Cardiovascular Angiography and Interventions and American Academy of Pediatrics (26). The registry was designed to capture all catheterization procedures performed nationwide within the United States (US) in pediatric and adult patients with congenital heart disease and has now more than 100 participating centers, resulting in important data on procedure-related adverse events (27,28).
The Interventional Armamentarium
General Considerations
The spectrum of transcatheter procedures available for the treatment of children and adults with congenital heart disease has rapidly increased over the last three decades. While the technical skills of the operator combined with a sound anatomic and hemodynamic understanding of a patient’s condition remain without a doubt the most important ingredients for successful outcome of an interventional catheter procedure, the choice of the appropriate equipment is almost equally as important for a successful outcome. With rapid progress that is being made in the development of new and more refined equipment, the operator has an inherent responsibility to keep up-to-date with these development efforts and to avoid procedural failures in situations where the use of a different type of equipment may lead to a very different outcome. Even though many interventional meetings have a focus on new device developments, the choice of appropriate balloons, catheters, sheaths and wires is in many situations even more important for a successful outcome. It is beyond the scope of this discussion to describe all available balloon catheters, but the operator has to make a well-informed decision on which balloon to use, based on profile, rated maximum pressure, available lengths, and degree of compliance and adjust his/her choice to suit specifically the therapeutic intervention that is intended. For example, balloon-in-balloon (BIB) catheters (NuMED, Hopkinton, USA) are specifically suited for stent deployment, while the family of high-pressure Mullins balloons (NuMED, Hopkinton, USA), or ultra–high-pressure Atlas balloons (Bard Peripheral Vascular Inc., Tempe, AZ, USA) aids exquisitely when high-pressure balloon angioplasty or stent re-expansion to a larger diameter is required.
Even though transcatheter devices have long been available for the management of congenital cardiac lesions, the greatest progress has been made through introduction of a large variety of newer devices that were specifically developed for individual congenital cardiac lesions over the last 10 years. This progress has enabled many procedures to be safely performed in a much wider range of clinical centers. In this chapter, a variety of device-specific sections have been taken with permission from an article on this topic that was published in “Expert Review of Medical Devices” (29).
The following device section is centered on transcatheter devices that are presently approved or investigated within the US, and includes a discussion of devices available for the occlusion of septal defects as well as occlusion of vascular structures. The spectrum of devices that are discussed below is not intended to be complete, but rather represents subjective choices of the authors. Devices for the treatment of structural cardiac lesions or the treatment of acquired heart disease are not included in this chapter. Since the last edition of this textbook, very few devices were newly approved in the US for the treatment of congenital cardiac lesions.
Devices for Occlusion of Septal Defects
The development of transcatheter devices for the occlusion of septal defects has been progressing at a rapidly accelerating pace, ever since King and colleagues first described a percutaneous technique to close ASDs (5). Various devices are presently approved by the FDA, either for regular use or under a humanitarian device exemption (HDE), while others are presently being evaluated in clinical trials. Even though devices are usually developed to address a specific type of septal defect, it is not uncommon that they are also used for occlusion of other types of defects on an “off-label” basis, once regular premarket approval (PMA) has been obtained.
The AMPLATZER Septal Occluder gained FDA approval in December 2001 for occlusion of secundum ASDs as well as fenestrations after surgical completion of a Fontan-type circulation. It is presently the most frequently used transcatheter device for occlusion of septal defects within the US and worldwide. Modifications of the principle device design have since been developed to accommodate the specific requirements of patent foramen ovale (AMPLATZER PFO Occluder), multifenestrated ASD (AMPLATZER Cribriform Septal Occluder), muscular VSD (AMPLATZER Muscular VSD Occluder), perimembranous VSD (AMPLATZER Membranous VSD Occluder), as well as post myocardial infarction VSD (AMPLATZER Muscular VSD [Post Myocardial Infarction] Occluder), albeit the latter two devices not being approved in the US. The AMPLATZER Muscular VSD Occluder is approved for use in “high-risk” muscular VSD. All of the AMPLATZER devices are manufactured by St. Jude Medical, St Paul, MN.
The CardioSEAL ASD occlusion device (Nitinol Medical Technologies, Boston, MA) was developed as a successor to the Clamshell device. At the same time as the AMPLATZER Septal Occluder was approved, the CardioSEAL device gained regular use approval by the FDA for occlusion of “high-risk” muscular VSDs, under a registry requirement. Furthermore, it gained HDE approval for occlusion of patent foramen ovale (PFO) with associated recurrent stroke in patients who were taking therapeutic Warfarin. A self-centering modification of the CardioSEAL device, the STARFlex Occluder has been evaluated for the treatment of PFO and stroke (CLOSURE I) (30,31). Neither of those devices is still marketed within the US.
Another device that has acquired FDA PMA for the occlusion of ASD is the HELEX Septal Occluder (W.L. Gore & Associates, Flagstaff, AZ) (32), and a modification of the original device is presently being evaluated in clinical trials. A variety of other devices
have been used outside the US but have not gained FDA approval (33,34,35,36).
have been used outside the US but have not gained FDA approval (33,34,35,36).
Devices for Occlusion of Vascular Structures
Porstmann et al. (3) introduced a technique of transcatheter closure of the ductus arteriosus in 1967. The procedure was complicated and required a large arterial cannulation and as a result, this technique never found widespread use.
Rashkind and Cuaso, while still working on the septostomy balloon, also developed a device for closure of the patent ductus. This device was a small umbrella that attached to the ductus by tiny hooks at the ends of the umbrella arms. The first successful use of this early device was reported in 1979 (37). It was modified into a double umbrella, which fixed in the ductus by a spring mechanism of the arms expanding against the vessel walls. Even though the device had undergone extensive trials, resulting in more than 700 prospectively monitored PDA occlusion procedures performed in the US, it never quite made it to regular use approval (38,39). However, the extensive experience gained in this process formed the basis upon which virtually all subsequent devices have been developed.
A large variety of devices have been developed to facilitate occlusion of vascular structures. Embolization coils have been used by general interventional radiologists for almost three decades (4). However, it was not until the 1980s that these were introduced into the interventional armamentarium of the pediatric cardiologist, initially for occlusion of abnormal collateral vessels (40), and subsequently in 1992 for the occlusion of the patent arterial duct in children (41). Gianturco coils are available in a variety of sizes but the lack of a controlled release mechanism ultimately stimulated the development of the Jackson coils, which are presently only available outside the US (42), and its US counterpart, the Flipper coil (Cook, Bloomington, IN). An MRI compatible modification of the Gianturco and Flipper coils, the MReye coil (Cook, Bloomington, IN) was introduced in 2006, and has since evolved as the most commonly used coil to date, mainly due to the notably lower incidence of MRI artifact (43).
Even though coils were and still are used “off-label” for the occlusion of the patent arterial duct, it was not until 2003 that a custom-made device designed specifically for the occlusion of the PDA gained FDA PMA (44). The AMPLATZER Duct Occluder (ADO; AGA Medical Corporation, Golden Valley, MN), which was first introduced into clinical use in 1997, as the first device approved specifically for this indication (44). However, a modification of the device, the ADO II, a double disc device, (St. Jude Medical, St Paul, MN) has since been approved for PDA closure (45,46,47). In addition, the Nit-Occlud PDA occlusion device (pfm AG, Cologne, Germany), a modification of the Duct-Occlud device, has been approved for PDA closure (48,49). An additional “coil modification,” the Gianturco-Grifka Vascular Occlusion Device (Cook, Bloomington, IN), has gained also FDA approval, even though its cumbersome delivery technique has prevented its widespread use. A large variety of additional coils are available for regular use, such as Target coils (Target therapeutics, Fremont, CA), Tornado coils (Cook, Bloomington, IN) as well as Nester coils (Cook, Bloomington, IN). However, these are less frequently used in congenital cardiac interventions and are therefore will not be further discussed in this review.
Further additions to the interventional armamentarium include the AMPLATZER Vascular Plug, which was first described in 2003, and which has since acquired regular use approval for peripheral arterial and venous embolizations (50). Modifications of this device, the AMPLATZER Vascular Plug II and Vascular Plug IV (St. Jude Medical, St Paul, MN), have since gained PMA approval. Other devices are available internationally, but not approved in the US.
Endovascular Stents
Charles E. Mullins, MD pioneered the introduction of endovascular stents into the armamentarium of the congenital cardiac interventionalist in the late 1980s and early 1990s (8,51). The most common indications for stent placement include rehabilitation of branch pulmonary artery stenosis as well as treatment of primary and recurrent coarctation of the aorta or aortic arch obstructions. However, stents are also used to rehabilitate stenotic lesions in systemic and pulmonary veins, and to maintain patency of structures that would otherwise close, such as the arterial duct or a foramen ovale. Endovascular stents are particularly helpful in locations that are either inaccessible to surgical techniques, or where the scarring resulting from surgical intervention is unlikely to achieve an improvement of the lesion, which applies to thin-walled vessels such as distal pulmonary arteries or pulmonary veins.
Virtually all approved stents that are used in the US in transcatheter therapy of congenital cardiovascular lesions have not been designed specifically for these indications, and are therefore used on an “off-label” basis. The choice of which stent to use for a particular lesion, depends not only on age and size of the patient, but also on expected adult dimensions of the vascular structure that is being treated, the morphology of the specific lesion, the presence of side branches that need to be crossed, expected future surgical procedures as well as previous surgical and transcatheter procedures and their outcome.
An ideal stent would combine a variety of characteristics, which are often exclusive to each other and may require opposing design goals:
Low profile that allows introduction through small delivery sheaths.
Easy crimpability or availability as premounted stents.
Possibility for re-expansion with maximum achievable diameter being sufficient to accommodate the growth of a vessel to adult size.
High degree of flexibility for placement around curved structures.
Allow rehabilitation of vessels that are overlapped by the placed stent through the stent meshwork/cells (e.g., open cell design).
High radial force to accommodate very tight and scarred lesions.
Rounded atraumatic edges that avoid damage to the vessel and the balloon upon which it is mounted.
Nonexisting or low degree of stent shortening during expansion.
Stent material that is MRI compliant, noncorrosive, and does not lead to increased blood levels of metal.
Low risk of neointimal proliferation, possibly through internal coating.
Possibility of biodegradable material with a platform to sustain drug coating to minimize tissue reaction.
Unfortunately, an ideal stent does not exist; therefore a careful decision has to be made on which to use. Dr. Charles Mullins always emphasized that the interventional cardiologist should not create a later surgical stenosis by failure of the stent to be able to be dilated to the adult-sized diameter of the vessel. However, it is important to note that the use of premounted and smaller-diameter stents as a “palliative” procedure to relieve critical vascular narrowing in small infants and children, who will have later surgery as a “staged repair” or conduit change, is now a very important treatment option. When judging the suitability of stent implantation, one always has to remember that suboptimal balloon angioplasty may result in impaired interval growth of the pulmonary arterial tree. Furthermore, recent studies have shown that small-diameter stents can be intentionally fractured when necessary with the use of high-pressure balloons (52). In addition, surgeons are well equipped to excise or patch a stent when necessary (53).
Table 17.1 Stents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 17.1 summarizes the most important characteristics of those larger diameter stents that are presently most frequently used in congenital heart disease in the US. The Cheatham-Platinum (CP) stent (NuMED, Hopkinton, NY), as well as its covered variety, is available in the US for compassionate and investigational use (COAST II trial) only, but is approved outside the US as the only stent specifically approved for the treatment of congenital heart disease, and has favorable design characteristics for the use in native and recurrent coarctation. Covered stents are especially useful for the treatment of ruptured vessels, including aortic aneurysms. Even though the early versions of the Palmaz stent (P108, P188, P308) (Johnson & Johnson, Warren, NJ), as well as the “ITI Double Strut” and “Double Strut LD” stent (Intra Therapeutics Inc., St Paul, NM) are still available, they have largely been replaced by the Genesis XD stents (Cordis, Warren, NJ), as well as the EV3 Mega LD and Max LD stents (EV3, Plymouth, MN), and are therefore not listed in this summary. Smaller-diameter stents that are used in the congenital pediatric population include self-expandable Nitinol stents, the premounted Palmaz Blue stents (Cordis, Warren, NJ), the premounted Cook Formula 418 stents (Cook, Bloomington, IN), as well as various coronary stents.
The use of intravascular stents has provided a definitive solution to the problem of overdilation that is frequently required when performing standard balloon angioplasty. There has been extensive favorable experience and up to 15 years’ follow-up in patients with pulmonary artery branch stenosis and systemic vein stenosis. In the single-center series of Charles E. Mullins and associates at Texas Children’s Hospital, more than 655 stents were implanted in 340 patients with pulmonary artery and systemic vein stenoses. The largest group of patients in this series had lesions involving the central pulmonary arteries in postoperative patients and postoperative central systemic vein or systemic venous baffle stenosis. Many of these stenotic veins had a totally occluded initial lumen; some of the venous channels were purposely perforated with a wire or long needle. The mean vessel diameter increased from 5 to 12 mm, and there was lasting success, with less than 0.5% showing restenosis during the follow-up period. The number of complications from the procedure or the stents themselves was minimal.
Percutaneous Valves
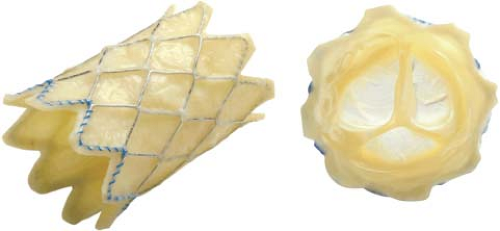
Since Dr. Phillip Bonhoeffer performed the first transcatheter pulmonary valve replacement in a human in 2000 (9), several thousand of these procedures have been performed worldwide. In January 2010, the Melody valve (Medtronic, Minneapolis, MN) gained FDA HDE approval in the US (54,55). Its use is indicated for dysfunctional right ventricular outflow tract (RVOT) conduits larger than 16 mm, even though the valve has also been used for other indications (56,57). The Melody transcatheter pulmonary valve is a bovine jugular venous valve that is sutured within an 8-zig, 28-mm Covered CP stents (Fig. 17.1). The valve is mounted on an 18-mm, 20-mm, or 22-mm BiB balloon, which is part of the specifically designed Ensemble delivery system.
In addition to the Melody valve the Sapien valve (Edwards Lifescience, Irvine, CA, USA) has been approved for the treatment of dysfunctional conduits. The valve is mounted within a stainless steel stent and comes in diameters of 23 and 26 mm, and as such can be used for larger conduits that would not be suitable for implantation of the Melody valve. However, prestenting is required to create a landing zone, due to the very short height of 14.5 to 16 mm (58).
How to Create, Enlarge, and Maintain Intra-Atrial Communications
Balloon Atrial Septostomy
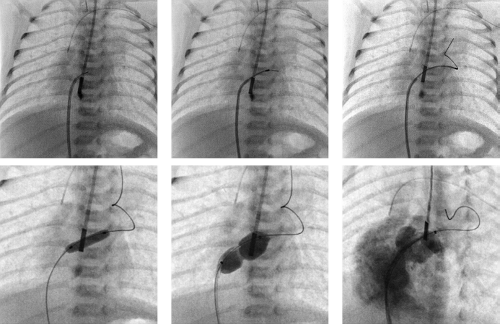
Balloon atrial septostomy (BAS), introduced by Rashkind et al. (2) in 1966, is a lifesaving procedure and one of the few remaining indications for an emergency catheterization in infants. BAS should be available in every institution that cares for infants with congenital heart disease. Because of septal thickening with age, BAS is consistently effective only in infants younger than 1 month of age. The BAS procedure is indicated in all infants with simple transposition of the great arteries (TGA) who are younger than 1 month of age
with a restrictive intra-atrial communication, and not otherwise scheduled for immediate surgical correction. Emergency BAS is imperative in any infant with simple TGA who exhibits evidence of acidosis as a result of an inadequate intra-atrial communication. This procedure also may be indicated for palliation in other congenital heart lesions in equally young infants, in whom all systemic, pulmonary, or mixed venous blood must traverse through a restrictive intra-atrial communication to return to the active circulation. These lesions include those complex single-ventricle defects associated with hypoplastic right or left ventricles and some instances of total anomalous pulmonary venous connection. BAS is rarely indicated in cases of pulmonary valve atresia and intact ventricular septum. It can be extremely hazardous in left-sided heart hypoplasia if the left atrium is diminutive, as there is a heightened risk of perforation or avulsion of atrial appendage or pulmonary vein. In such cases, static balloon dilation of the atrial septum may be preferable (59).
with a restrictive intra-atrial communication, and not otherwise scheduled for immediate surgical correction. Emergency BAS is imperative in any infant with simple TGA who exhibits evidence of acidosis as a result of an inadequate intra-atrial communication. This procedure also may be indicated for palliation in other congenital heart lesions in equally young infants, in whom all systemic, pulmonary, or mixed venous blood must traverse through a restrictive intra-atrial communication to return to the active circulation. These lesions include those complex single-ventricle defects associated with hypoplastic right or left ventricles and some instances of total anomalous pulmonary venous connection. BAS is rarely indicated in cases of pulmonary valve atresia and intact ventricular septum. It can be extremely hazardous in left-sided heart hypoplasia if the left atrium is diminutive, as there is a heightened risk of perforation or avulsion of atrial appendage or pulmonary vein. In such cases, static balloon dilation of the atrial septum may be preferable (59).
The preferable approach for performing a BAS is percutaneous through the femoral vein. In addition, balloon atrial septostomy can be accomplished successfully using an umbilical venous approach. For acute, temporary palliation, many of these procedures can be performed under echocardiographic guidance in the neonatal intensive care unit, but whenever possible, the availability of fluoroscopy in the cardiac catheterization laboratory adds an additional safety margin to the procedure. BAS catheters are available from variety of manufacturers and in different designs. The classical Miller–Edwards balloon septostomy catheter (Edwards Lifescience, Irvine, CA), is a single-lumen catheter with a fairly compliant latex balloon at the end, that is rated up to 4 cc capacity, but can be inflated with larger quantities if required. It requires the use of a 7-Fr sheath and is still in widespread use, even though newer catheter varieties offer more favorable balloon characteristics. Because of the single lumen it cannot be tracked over a wire and the fairly high compliance often requires large balloon inflations to successfully perform a septostomy, which is a considerable disadvantage especially in smaller infants under 3 kg. Other catheter varieties include the USCI Rashkind balloon catheter (USCI, Glens Falls, NY), which have a 6-Fr shaft, as well as the newer NuMED Z5 atrioseptostomy catheters (NuMED, Hopkinton, NY) that are available on a 4- or 5-Fr shaft and can be passed through a 5- or 6-Fr introducer. These balloons have the advantage of being noncompliant at inflation volumes of 1 or 2 mL, which is very important when attempting to tear, rather than stretching the atrial septum. The balloons also have the additional benefit of being able to be passed over a wire.
Once the deflated balloon catheter is introduced into the venous system and while it is observed on fluoroscopy or by echocardiography, it is advanced through the right atrium and through the foramen ovale or small ASD into the left atrium. While continually observed on fluoroscopy and/or two-dimensional echocardiography, the balloon is inflated with dilute contrast to the maximum diameter of the balloon or, in the smaller atrium, to the maximum diameter tolerated within the particular left atrium. It is essential to determine that the balloon is completely free within the left atrium before initiating the “jerk” across the septum. Failure to do so can result in laceration or even separation of the left atrium from the pulmonary veins. The balloon is pulled rapidly or, better stated, “jerked” across the atrial septum into the right atrium using as forceful and rapid, but at the same time, as short and controlled a pull, as possible. Especially when using the fairly noncompliant NuMED atrioseptostomy catheters it is important to avoid pulling the balloons into the inferior vena cava (IVC), as the rapid “jerk” can easily create a laceration or disruption of the IVC-RA junction. The entire procedure should be performed one to four times or until no resistance to withdrawal of the fully inflated balloon is encountered or until enlargement of the defect and looseness or “flipping” of the septum primum tissue are documented by echocardiography. Following a successful septostomy, there should be an immediate equalization or near equalization of pressures across the atrial septum. Performed carefully with precise attention to details, the procedure carries only a small risk; yet it has the potential for a dramatic improvement in the infant’s hemodynamic and symptomatic status.
Blade Atrial Septostomy and Balloon Atrial Septoplasty
In infants over 1 month of age, and certainly for older children who might require an atrial septostomy for palliation of their cardiac defect, the atrial septum usually is frequently too tough or thick for a simple BAS to tear the septum. In 1975, Park et al. introduced the Park Blade Septostomy Catheter (Cook, Inc., Bloomington, IN, USA) and the blade atrial septostomy procedure to obviate this difficulty. A collaborative study from 1978 to 1982 (60) demonstrated the safety and effectiveness of the blade procedure. The indications for blade atrial septostomy are the same as considered for a balloon septostomy or for surgical atrial septostomy that otherwise would be needed in the older infant.
Blade catheters are available with three different blade lengths: 1.0, 1.34, and 2.0 cm. The two smaller blades (the PBS 100 and 200) are available on a 6-Fr catheter, and the 2.0-cm blade (the PBS 300) is on an 8-Fr catheter. Both blade catheter sizes require a sheath one size larger than the catheter shaft for smooth introduction.
The most consistent method of delivering the blade into the left atrium is to pass a long Mullins sheath over a catheter or dilator from the femoral vein through the right atrium, through the septum (either through the PFO or through a transseptal puncture), and into the left atrium. The blade catheter is advanced through this sheath, and the sheath is withdrawn well into the IVC. The blade is opened
carefully in the left atrium while it is continuously observed on fluoroscopy. Transesophageal echocardiographic guidance can add an additional safety margin to this procedure. The tip is directed anteriorly and to either the patient’s right or left side. In contrast to the balloon septostomy, the blade catheter is withdrawn slowly in a controlled but at the same time, forceful maneuver until the blade snaps through the septum. The “blading” is repeated four to eight times while changing the angle of extension of the blade as necessary and changing the blade direction from side to side until there is no further resistance to the withdrawal of the fully opened blade catheter.
carefully in the left atrium while it is continuously observed on fluoroscopy. Transesophageal echocardiographic guidance can add an additional safety margin to this procedure. The tip is directed anteriorly and to either the patient’s right or left side. In contrast to the balloon septostomy, the blade catheter is withdrawn slowly in a controlled but at the same time, forceful maneuver until the blade snaps through the septum. The “blading” is repeated four to eight times while changing the angle of extension of the blade as necessary and changing the blade direction from side to side until there is no further resistance to the withdrawal of the fully opened blade catheter.
The blade septostomy is followed by a balloon septostomy. In most patients, this can be accomplished using the Rashkind balloon technique; however, in larger or older patients, when the septum is tough or resistant to tearing, the blade incision can be extended by the use of static dilation balloons placed in the defect and inflated. Alternatively, balloon dilation alone after transseptal placement of a guidewire can be effective in creating or enlarging an ASD. The resultant defect will be somewhat smaller than the balloon or balloons used for dilation, so the balloon catheters must be oversized relative to the final defect diameter desired. As a result of the combined blade and ballooning, equalization of pressures between the two atria as well as a measurable increase in the mixing of the systemic and pulmonary venous blood should occur. In most cases, an adequate and permanent ASD is created, palliating the patient indefinitely or until a more permanent correction is possible. Stenting of the atrial septum has been performed in a few cases to ensure a lasting opening. The blade atrial septostomy can be accomplished in patients of any age or any size. Prior to the introduction of the transhepatic approach congenital absence or acquired blockage of the IVC had been the only absolute prohibition to a blade atrial septostomy.
With the availability of larger cutting balloons of up to 8 mm in diameter (Boston Scientific, Boston, MA), the combination of static cutting balloon septoplasty, followed by the use of larger diameter static balloons or standard balloon atrial septostomy, has become an important alternative to blade atrial septostomy in patients with a thickened atrial septum. The smaller the pre-existing septal defect, the higher the likelihood that the use of cutting balloon will achieve an adequate result (Fig. 17.2). This is especially important if the patient size is rather small or prohibitive to use of even a small PBS 100 blade catheter. If the existing intra-atrial communication is stretched then cutting balloon septoplasty may be unfeasible, and it may be more beneficial to perform a transseptal puncture to start with a “fresh” diminutive opening to facilitate a better result of cutting balloon atrial septoplasty. More technical details are provided in the section on Hybrid palliation of hypoplastic left heart syndrome (HLHS), where atrial septal interventions are particularly important and challenging (59).
Transseptal Puncture
Access to left heart structures is required at times to obtain accurate left atrial pressure recordings, or to facilitate interventional procedures such as the creation or closure of an intra-atrial communication
or balloon mitral valvuloplasty. In addition, access to left heart structures from a venous approach avoids the use of larger sheaths in the femoral artery, which can be especially beneficial in small children and infants. Most catheterization laboratories use the standard Brockenbrough transseptal needle, while the use of radiofrequency (RF) energy has added a more controlled technique specifically for infants with small left atria (61).
or balloon mitral valvuloplasty. In addition, access to left heart structures from a venous approach avoids the use of larger sheaths in the femoral artery, which can be especially beneficial in small children and infants. Most catheterization laboratories use the standard Brockenbrough transseptal needle, while the use of radiofrequency (RF) energy has added a more controlled technique specifically for infants with small left atria (61).
The Brockenbrough needle is available in sizes of 62 and 72 cm of usable length, and is usually used in conjunction with a transseptal Mullins introducer set (Cook, Bloomington, IN). A fairly stiff exchange length wire is placed in superior vena cava (SVC) or preferably innominate vein, and the Mullins sheath and dilator are advanced to a position within the SVC. The wire is withdrawn and the transseptal needle is advanced through the sheath to a position just 1 to 2 mm below the tip of the dilator. On occasions difficulty can be encountered when introducing the transseptal needle through the hub or dilator and sheath, at which point the two components should be are separated temporarily by 1 to 2 cm to allow passage of the needle through the hub. Once the needle has been positioned appropriately, the whole system needs to be flushed and the needle connected to a pressure monitoring system. There is usually a 1 to 2 cm separation between the needle and the hub of the dilator and care has to be taken to maintain this distance throughout the procedure. Needle, sheath, and dilator are then removed carefully as one unit with the system being gently pointed toward the patient’s left scapula (and posterior) when withdrawing from the SVC and sliding along the atrial septum. Any harsh movement or torque should be avoided at this stage as it can create injury to adjacent vessel or chamber walls. Once the unit has passed about 2/3 of the atrial septal length inferiorly, one often notices the tip of the dilator suddenly moving slightly to the left while advancing into the fossa ovalis. At this stage, sheath dilator and needle are withdrawn inferiorly for a further few millimeters just below the limbus of the ovale fossa. At this point, sheath and dilator are fixed while the needle is advanced slightly out of the tip of the dilator until it fully engages the dilator. At this point the whole unit is advanced while carefully observing the recorded pressure tracing, and maintaining a left and posterior direction. The operator usually feels a slight “pop” when the needle traverses the atrial septum and this should be followed by the emergence of left atrial pressure tracing. If any untoward resistance or inappropriate pressure tracings appear, the operator should stop any advances of needle, sheath, and dilator. If a position is unclear, a small amount of contrast can be instilled through the needle. If a left atrial pressure tracing is obtained the entire system is advanced slightly further toward the patient’s left to allow at least the proximal portion of the dilator to pass through the atrial septum. This is performed in very diminutive steps while maintaining careful observation for left atrial pressure tracings. At this point, the needle is withdrawn just inside the dilator to add additional stiffness to the system and the Mullins sheath is advanced over the dilator and needle across the atrial septum into the left atrium. If at any stage during the procedure doubt about the accurate position occurs, then the system is either withdrawn in very small steps until appropriate pressure recording re-occur, or small amount of contrast is injected to confirm the sheath’s location.
An alternative to the use of the classical Brockenbrough needle is the use of RF energy. At the present time, two techniques of RF perforation of the atrial septum are available, depending on patient and left atrial size. In larger patients, the Toronto transseptal catheter can be used in combination with the 8-Fr Torflex transseptal sheath and dilator (Both: Baylis Medical Corporation, Montreal, Quebec, Canada). The Toronto transseptal catheter is curved at the end by about 210 degrees to avoid continued perforation of adjacent structures once the atrial septum is traversed. It also has a slightly increased stiffness when compared to the Nykanen RF perforation wire (Baylis Medical Corporation, Montreal, Quebec, Canada) that is specifically suited to allow tracking of the transseptal sheath across the perforated atrial septum. Initial positioning of the transseptal sheath is very similar to the Brockenbrough transseptal technique. However, instead of using a stiff and forceful needle to traverse the atrial septum, low-power, and high-intensity electrical current is used to allow the transseptal catheter to advance through the atrial septum, usually with minimal force and a much lower risk of injuring adjacent structures.
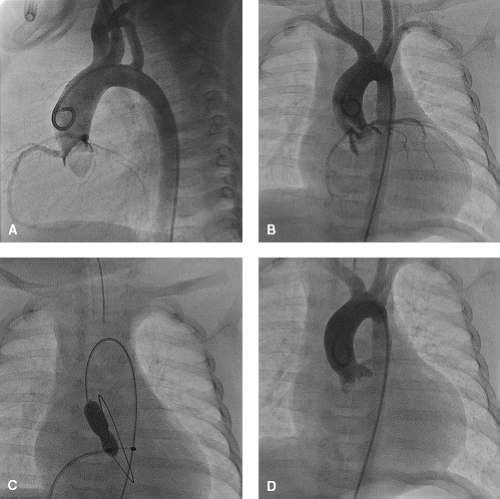
In small infants, especially in neonates with a small left atrium, the curve of the Toronto transseptal catheter is too large to fit snugly into the small left atrium. Therefore in these patients, a 5-Fr JR catheter is used to obtain an appropriate position along the atrial septum to facilitate RF puncture (Fig. 17.2). An 180-cm 0.035-inch outer diameter coaxial injectable catheter (Baylis Medical Corporation, Montreal, Quebec, Canada) is loaded over a 260-cm 0.024-inch Nykanen RF perforation wire and the RF wire is advanced to the tip of the positioned JR catheter. The use of transesophageal echocardiography (TEE)-guided perforation of the atrial septum has greatly improved the safety and success of this challenging procedure. In infants less than 3 kg placing the 8-Fr intracardiac echocardiography (ICE) catheter (Acuson-Siemens Co., Mountain View, CA, USA) transesophageally has been suggested by Hill and colleagues as an additional guidance of the transseptal puncture (62). Once an accurate position is confirmed with hand injections of contrast through the side port of a Touhy Borst adapter, RF energy is applied, while also exerting a gentle push on the RF wire. Once perforation has occurred, an orientating injection of contrast can be performed through the Touhy Borst adapter, before advancing the coaxial catheter over the RF wire across the perforated lesion. The RF wire is then exchanged to an appropriate exchange-length wire that can be preshaped according to the size of the left atrium. This positioned wire then facilitates cutting balloon septoplasty, possibly followed by balloon atrial septostomy or standard septoplasty using larger balloon diameters, depending on the size of the intra-atrial communication that is required.
Balloon Aortic Valvuloplasty
The possibility of creating significant aortic regurgitation has always been the main concern when considering balloon dilation of congenitally stenotic aortic valves, especially in infants and small children. In 1984, Lababidi and colleagues reported for the first time on a series of 23 patients with congenital aortic valve stenosis, in whom the procedure was documented to be safe and effective (31). Despite this report, general acceptance of the technique was relatively slow. One of the fundamental problems of the procedure remains the risk of creating significant aortic insufficiency, which then may accelerate the need for any surgical aortic valve procedure. While this is less of a concern in the adolescent, where all other treatment options are available in such a situation, the problems are more significant in the infant who has a moderate degree of aortic valve stenosis, where severe aortic regurgitation may require a surgical procedure to be performed at an age where one would have otherwise preferably waited a little longer for the patient to grow.
Several other centers have demonstrated that the results of balloon aortic valve dilation approximated the results of surgical valvotomy but with less risk and much less morbidity. However, the disease has very wide morphologic variations, ranging from the critical ill neonate with borderline LV and left ventricular outflow tract (LVOT) size and a very dysplastic aortic valve, to the young adult with isolated valvar stenosis and well-formed aortic valve.
The decision when to take a patient with congenital aortic valve stenosis to the catheterization laboratory is not always straightforward. A variety of factors have to be considered including peak and mean Doppler gradients, age and gender, EKG findings, LV function and degree of LVH, symptoms, exercise tolerance and desire to exercise competitively, valve morphology and pre-existing aortic insufficiency, as well as associated lesions such as coarctation or
mitral valve (MV) disease. Guidelines for the treatment of congenital aortic valve stenosis in children are derived from the adult population (63), where a peak-to-peak gradient in excess of 60 mm Hg in asymptomatic patients is considered an indication for transcatheter intervention. However, in symptomatic patient, or with the presence of ischemic or repolarization changes on EKG, a gradient of 50 mm Hg should be used. However, peak systolic gradients are only meaningful if left ventricular function is normal. Documented aortic valve stenosis in the critically ill neonate with a dilated left ventricle and poor left ventricular function, would be considered a candidate for transcatheter intervention irrespective of any obtained transvalvar gradient, and probably represents one of the few true emergency transcatheter interventions in congenital heart disease.
mitral valve (MV) disease. Guidelines for the treatment of congenital aortic valve stenosis in children are derived from the adult population (63), where a peak-to-peak gradient in excess of 60 mm Hg in asymptomatic patients is considered an indication for transcatheter intervention. However, in symptomatic patient, or with the presence of ischemic or repolarization changes on EKG, a gradient of 50 mm Hg should be used. However, peak systolic gradients are only meaningful if left ventricular function is normal. Documented aortic valve stenosis in the critically ill neonate with a dilated left ventricle and poor left ventricular function, would be considered a candidate for transcatheter intervention irrespective of any obtained transvalvar gradient, and probably represents one of the few true emergency transcatheter interventions in congenital heart disease.
Balloon aortic valvuloplasty is now considered a standard technique performed in virtually any center that offers interventional treatment for congenial cardiac lesions (Fig. 17.3) and the AHA guidelines list critical aortic valve stenosis (regardless of gradient), isolated aortic valve stenosis with a peak-to-peak systolic transcatheter gradient in excess of 50 mm Hg, as well as a gradient in excess of 40 mm Hg when combined with symptoms or EKG changes as class I indications for transcatheter balloon aortic valvuloplasty (10). However, there are proponents of a surgical approach to congenital aortic valve stenosis, especially with newer surgical techniques, and many articles often report comparable results (64).
In contrast to balloon pulmonary valvuloplasty, where the vast majority of patients can be expected not to require any further transcatheter or surgical intervention, aortic valvuloplasty is usually palliative in nature, and not infrequently aimed at delaying an inevitable surgical procedure, be it valve replacement or Ross procedure, until a time when the child has reached close-to-adult size. A surgical series reporting on the results of surgical aortic valvuloplasty documented a freedom from aortic valve replacement (AVR) of 72% at 10 years, and 60% at 18 years (65). This is very similar to the results of transcatheter aortic valvuloplasty where a freedom from AVR of 79% at 10 years and 53% at 20 years has been reported (66).
In general, aortic valve dilation is performed retrograde with a catheter introduced into the femoral artery. While an antegrade approach with transseptal puncture offers a slight advantage in maintaining a centered balloon position across the aortic valve during balloon inflation, the technique is more cumbersome and is associated with risk of injuring the MV apparatus with resulting mitral insufficiency, and is therefore not routinely employed. An end-hole catheter is passed from the femoral artery across the aortic valve to a stable position in the left ventricle. A double-balloon technique with the introduction procedure repeated from both femoral arteries may offer advantages for the aortic valve dilation in selected older patients, even though today, the spectrum of available balloons usually allows a successful employment of a single-balloon technique.
The catheter/wire passage retrograde across the stenotic aortic orifice is the most difficult maneuver in the entire procedure, and therefore should ideally be performed only once during the procedure. Before crossing the aortic valve, an aortogram with 25-degree LAO/cranial angulation of the AP and straight lateral projection should initially be performed to exactly delineate the size of the aortic valve annulus, while at the same time demonstrating any angiographic evidence of pre-existing aortic valve insufficiency. The exact technique for passing the wire or catheter into the left ventricle varies
from operator to operator. A Judkins right coronary catheter curve or multipurpose catheter is used by some operators with success in crossing the aortic valve from this approach. However, the Judkins left coronary catheter may offer advantages in many patients, as the curvature is automatically directed to the leftward and posterior opening of the congenitally stenotic aortic valve. Once the valve is crossed, an end-hole catheter (not Judkins left) is advanced over the wire into the left ventricle, and the wire replaced with an extra stiff exchange-length wire with a long floppy tip, which is looped within the ventricle to protect the ventricular apex from perforation by the catheter tip and to minimize ventricular ectopy. A left ventricular angiogram is optional. If an atrial communication is present, hemodynamic evaluation can be performed by advancing a catheter antegrade into the left ventricle with simultaneous pressure recording in the ascending aorta. The same approach is then also used for left ventricular angiography. In neonates and infants, a floppy-tipped coronary wire with a relatively stiff body may be advanced across the valve and allowed to loop in the left ventricle. Care has to be taken to prevent the wire from being ejected from the left ventricle and therefore, once positioned, wire control should be maintained throughout the procedure. By using a floppy-tipped, high torque guidewire, the wire does not need to be changed, and the first catheter to cross the valve can be the dilation balloon (thus minimizing the period of potential low output). The use of stiffer exchange wires and longer dilation balloons may aid in maintaining an exact position of the balloons across the valve during inflation and, in turn, eliminate the “shear” trauma to the valve from balloon movement during inflation. With the wire secured within the left ventricle, the deflated balloon is manipulated through arterial sheaths and passed retrograde over the wire. We do not believe that direct introduction of the balloon through the skin should have any role to play, as the balloon profile of balloons has considerably decreased, thereby allowing the appropriately sized balloon to be introduced through fairly small sheaths. In addition, it is quite conceivable that the pulling of a deflated balloon directly through the femoral and iliac arteries may cause more harm to the vessel than using the appropriately sized sheath. Once the balloon is positioned across the stenotic valve, the balloon is rapidly inflated to the recommended maximal pressure and then rapidly deflated.
from operator to operator. A Judkins right coronary catheter curve or multipurpose catheter is used by some operators with success in crossing the aortic valve from this approach. However, the Judkins left coronary catheter may offer advantages in many patients, as the curvature is automatically directed to the leftward and posterior opening of the congenitally stenotic aortic valve. Once the valve is crossed, an end-hole catheter (not Judkins left) is advanced over the wire into the left ventricle, and the wire replaced with an extra stiff exchange-length wire with a long floppy tip, which is looped within the ventricle to protect the ventricular apex from perforation by the catheter tip and to minimize ventricular ectopy. A left ventricular angiogram is optional. If an atrial communication is present, hemodynamic evaluation can be performed by advancing a catheter antegrade into the left ventricle with simultaneous pressure recording in the ascending aorta. The same approach is then also used for left ventricular angiography. In neonates and infants, a floppy-tipped coronary wire with a relatively stiff body may be advanced across the valve and allowed to loop in the left ventricle. Care has to be taken to prevent the wire from being ejected from the left ventricle and therefore, once positioned, wire control should be maintained throughout the procedure. By using a floppy-tipped, high torque guidewire, the wire does not need to be changed, and the first catheter to cross the valve can be the dilation balloon (thus minimizing the period of potential low output). The use of stiffer exchange wires and longer dilation balloons may aid in maintaining an exact position of the balloons across the valve during inflation and, in turn, eliminate the “shear” trauma to the valve from balloon movement during inflation. With the wire secured within the left ventricle, the deflated balloon is manipulated through arterial sheaths and passed retrograde over the wire. We do not believe that direct introduction of the balloon through the skin should have any role to play, as the balloon profile of balloons has considerably decreased, thereby allowing the appropriately sized balloon to be introduced through fairly small sheaths. In addition, it is quite conceivable that the pulling of a deflated balloon directly through the femoral and iliac arteries may cause more harm to the vessel than using the appropriately sized sheath. Once the balloon is positioned across the stenotic valve, the balloon is rapidly inflated to the recommended maximal pressure and then rapidly deflated.
One difficulty of the procedure is to keep the balloon positioned across the aortic valve during inflation. Once it is inflated, a balloon tends naturally to move toward the ascending aorta because of the ejecting forces created by the left ventricle. It is generally difficult to push against these forces (unless using an antegrade approach), but a longer balloon length aids this process. Adenosine has been used to achieve a temporary cardiac standstill, but its timing in relation to balloon inflation is often difficult to predict. A more controllable method of reducing the cardiac output is through rapid right ventricular pacing (67). The rate of pacing can be adjusted prior to balloon inflation to achieve a drop in blood pressure by at least 50% and these settings are then available to be used during the inflation process. An inflation device that can be operated using a single hand is preferential, as this allows the operator to use the other hand to maintain control of the balloon catheter, making very fine adjustments as the balloon is inflated. The balloon is then immediately and rapidly deflated, with the entire process taking no more than 5 to 10 seconds. Arterial pressures should be monitored throughout the procedure. To limit the potential damage to the aortic valve, only one inflation should be performed provided that the operator is assured that (a) the balloon remained properly positioned in the valve; (b) the balloon was of adequate size; and (c) the waist disappeared. Regardless of the technique, a marked drop in systemic pressure, a rise in left ventricular pressure, and resultant bradycardia may transiently result. The double-balloon technique using two balloons placed side by side across the valve may minimize this problem, but more importantly one has to avoid prolonged inflations with any technique when performing aortic balloon dilations. With successful valve dilation, after the balloon is deflated, both the blood pressure and heart rate should return spontaneously to normal.
For a single-balloon technique, the initial balloon is chosen with a diameter of about 80% to 90% of the measured aortic annulus diameter. After each set of inflations, the hemodynamic result and the degree of aortic insufficiency are evaluated. If no or only a mild change in the degree of aortic insufficiency has been observed with still a significant residual gradient (>35 mm Hg), repeat dilation valvuloplasty is done with a balloon sized just 1 to 2 mm above the one previously used. Brown and colleagues recently demonstrated that freedom from AVR was improved when comparing patients with a residual gradient of less than 30 mm Hg to those with a gradient in between 30 and 39 mm Hg (66). Even more importantly, freedom from AVR was better in patients with a gradient of less than 35 mm Hg but moderate to severe aortic regurgitation, when compared to those with more than 35 mm Hg but only mild aortic regurgitation, which suggests that the residual gradient may be more important when compared to aortic insufficiency than previously thought.
When using the double-balloon technique, the combined diameters of the two balloons should approximate 1.2 times the measured diameter of the aortic annulus. Because of the extensive manipulation in the left side of the heart and arteries, all these patients are systemically anticoagulated with heparin at the beginning of the procedure.
In the past, the most common complication of aortic balloon dilation was damage to the femoral arteries by the large balloon dilation catheters. This problem has been minimized by newer lower-profile balloon designs, use of the double-balloon technique where required, and diligent monitoring of ACT levels throughout the procedure. When arterial damage does occur, it usually can be managed medically or, rarely, surgically. In small infants, because of the increased risk of femoral artery injury from the introduction of the dilating balloon catheters into the vessels, several other approaches to aortic valve dilation have been described. The prograde approach, first passing a catheter, then a wire, and finally the balloon from the femoral vein to the right atrium, foramen ovale, left atrium, left ventricle, and prograde across the aortic valve is chosen by some. Although frequently successful, this approach has a high incidence of failure in delivering the balloons and, even more disturbing, a significant incidence of damage to the MV apparatus. Another approach is through a controlled cutdown on the carotid artery. As a result of extensive experience with extracorporeal membrane oxygenation (ECMO) and the safe introduction of cannulae into the carotid arteries, several centers, with the help of pediatric or vascular surgeons, dilate aortic valves in infants from this approach. The approach is direct to the aortic valve and requires less catheter manipulation and less overall time, and has resulted in no reported complications related to the technique. The ideal procedure for the small infant is still to be determined.
With a conservative dilation of the aortic valve, the gradient should be reduced to a gradient equal to or less than 35 mm Hg. This usually can be accomplished without inducing significant aortic insufficiency, no more than that seen after surgical valvotomy. Furthermore, as highlighted above, recent data by Brown and colleagues suggests that the residual gradient may be more important when compared to aortic insufficiency than previously thought, and reducing the gradient to less than 35 mm Hg may be more important, even if it were to come on the expense of moderate aortic insufficiency (66). However, the treatment approach has to be tailored to the individual patient and specifically in infants, gradient reductions to less than 40 mm Hg may be sufficient to delay the early need for aortic valve surgery. The long-term results, like surgical valvotomy, will be palliative; however, the catheter balloon dilation procedure is accomplished without a sternotomy or cardiopulmonary bypass with their inherent risks and morbidity. Balloon dilation of congenital aortic valve stenosis in pediatric patients and young adults is now the standard initial procedure for this lesion in most centers.
Balloon Pulmonary Valvuloplasty
With the development of the special, larger dilation balloons, a transcatheter technique for balloon pulmonary valve dilation was first introduced by Kan et al. (6) in 1982. The technique performed acutely was successful and, at the same time, carried little risk over and above the basic risk of a catheterization. By December 1986, 28 centers, voluntarily reporting to a collaborative registry (VACA), demonstrated the successful and safe application of the technique in more than 680 cases of pulmonary valve stenosis (68). With these data and many subsequent reports of successful use (69,70), balloon dilation has been accepted as the standard therapeutic procedure for pulmonary valvar stenosis. It is applicable to patients of all ages from the newborn period throughout adult life. With its excellent results and low rate of procedure-related complications, maximum instantaneous systolic Doppler gradients of as little as 35 mm Hg, when combined with evidence of right ventricular hypertrophy, should be considered an indication for balloon pulmonary valvuloplasty (71).
The degree of pulmonary valve stenosis is documented by accurate hemodynamic measurements in the catheterization laboratory. However, if the pulmonary valve is not easily crossed, then right ventricular angiography should be obtained prior to further attempts at crossing the valve.
The valve anatomy, size, and exact location are visualized angiographically, with standard AP (with some cranial angulation) and lateral being the most appropriate projections. Accurate determination of the valve annulus diameter is obtained using appropriate calibration techniques. With this information available, a long exchange guidewire is passed through an end-hole catheter into a distal pulmonary artery. The left pulmonary artery is preferable for this position because of the straighter course from the valve and main pulmonary artery to the left. However, in neonates with a patent arterial duct the wire may be passed through the duct into the descending aorta. The chosen wire should be fairly stiff to allow the balloon to track over the wire and across the stenosed pulmonary valve. In infants and smaller children, a stiff 0.018 wire with a floppy tip is frequently appropriate for this purpose. However, infants with critical pulmonary stenosis and a closed arterial duct may poorly tolerate placement of a wire or catheter across the valve; therefore the valve should only be crossed when all equipment has been prepared to immediately proceed with balloon pulmonary valvuloplasty.
McCrindle and colleagues documented that the optimum balloon diameter should be between 1.2 and 1.3 times the size of the pulmonary valve annulus for a “single-balloon” dilation (72). Lower balloon to valve annulus ratios are associated with an increased risk of recurrent or residual pulmonary valve stenosis, while ratios in excess of 1.4 are associated with an increased risk of clinically significant pulmonary insufficiency (72).
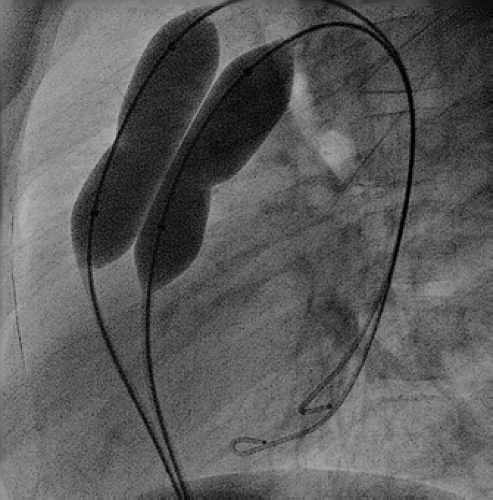
The choice of balloon catheters that can be used for this procedure is wide and depends to a degree on the individual valve morphology. In general, inflation pressures of more than six atmospheres are rarely necessary in patients with typical valve morphology, and therefore low-pressure balloons, such as Tyshak II (NuMED, Hopkinton, NY) with a lower profile are the preferred initial balloon types to be utilized. However, the maximum rated inflation pressures decline sharply when using the larger varieties of these balloons. Therefore high-pressure balloons, such as ZMed II (NuMED, Hopkinton, NY), or the double-balloon technique should be considered in these situations as the primary approach to balloon valvuloplasty (Fig. 17.4). High-pressure balloons may also be more beneficial when dealing with very dysplastic, thickened pulmonary valves in the older patient, or if there is associated supravalvar narrowing. In neonates with critical pulmonary valve stenosis and a closed arterial duct, a low-profile balloon with fairly rapid deflation characteristics such as the Tyshak II balloon should be used. Very low-profile balloons, such as the mini Tyshak balloons (NuMED, Hopkinton, NY), may cross the valve more readily, but their very slow deflation characteristics make these balloons an inappropriate choice in patients who have critical pulmonary stenosis without a patent arterial duct. If the valve cannot be crossed with the appropriate-sized balloon, smaller coronary balloons can facilitate predilating the valve to allow the larger balloon to be subsequently passed.
With the wire fixed in place in the distal pulmonary artery, the end-hole catheter is removed and the catheter with its deflated balloon is passed over this wire until the center of the balloon length is positioned exactly at the area of the stenotic valve. The balloon is then rapidly inflated to the pressure recommended by the manufacturer and is observed for the appearance of a circumferential indentation or “waist” in the balloon. Full inflation
results in disappearance of the waist. An inflation device that can be operated using a single hand is preferential, as this allows the operator to use the other hand to maintain control of the balloon catheter, making very fine adjustments as the balloon is inflated. The balloon is then immediately and rapidly deflated, with the entire process taking no more than 5 to 10 seconds. Arterial pressures should be monitored throughout the procedure. In contrast to balloon aortic valvuloplasty, more than one inflation is usually performed to assure the operator that (a) the balloon remained properly positioned in the valve; (b) the balloon was of adequate size; and (c) the waist disappeared early and at low pressures during subsequent inflations. When a single balloon is used, there is a significant drop in both systemic blood pressure and heart rate during inflation. With successful valve dilation, after the balloon is deflated, both the blood pressure and heart rate should return spontaneously to normal.
results in disappearance of the waist. An inflation device that can be operated using a single hand is preferential, as this allows the operator to use the other hand to maintain control of the balloon catheter, making very fine adjustments as the balloon is inflated. The balloon is then immediately and rapidly deflated, with the entire process taking no more than 5 to 10 seconds. Arterial pressures should be monitored throughout the procedure. In contrast to balloon aortic valvuloplasty, more than one inflation is usually performed to assure the operator that (a) the balloon remained properly positioned in the valve; (b) the balloon was of adequate size; and (c) the waist disappeared early and at low pressures during subsequent inflations. When a single balloon is used, there is a significant drop in both systemic blood pressure and heart rate during inflation. With successful valve dilation, after the balloon is deflated, both the blood pressure and heart rate should return spontaneously to normal.
To avoid the marked drop in systemic blood pressure and to reduce the trauma to the peripheral introductory veins, a double-balloon technique was introduced (73). This technique also allows the use of higher inflation pressures in patients with a large pulmonary valve annulus, where a single balloon would provide an inadequate rated burst pressure. The double-balloon technique uses two separate balloon catheters, each on a smaller shaft and with a smaller balloon “profile.” Each is introduced into a separate vein. With this technique, a second exchange wire is introduced from the opposite femoral vein and positioned across the pulmonary valve into a distal pulmonary artery, possibly next to the first wire. Two smaller-diameter balloon dilation catheters are advanced over the separate wires and centered in the valve orifice, and the two balloons are simultaneously inflated. Various formulae have been used to estimate the equivalence of double-balloon to single-balloon technique (71), ultimately resulting in comparison charts that allow choice of sizes of the two smaller balloons, depending on the size that would have been chosen if a single-balloon technique would have been used. However, a combined diameter of 150% to 160% of the pulmonary valve annulus can be used as a guide to choose the appropriate balloon sizes.
Reported success criteria for balloon pulmonary valvuloplasty have been variable. The VACA registry, dating back as much as 15 years, defined a gradient on follow-up of equal to or above 35 mm Hg as procedural failure (72). Holzer and colleagues defined procedural success as either a reduction of the peak systolic RV/MPA gradient to less than 25 mm Hg, or a reduction of the valvar gradient by at least 50%, or a reduction of the RV/systemic pressure ratio by at least 50% (74). However, with pure valve stenosis and a closed PDA, regardless of the initial gradient, one should expect to reduce the pressure gradient across the nondysplastic pulmonary valve to less than 10 mm Hg by balloon valvuloplasty in most patients, with an equivalent reduction in the RV to systemic pressure ratio.
If the initial results are suboptimal, the balloon size should be increased up to 130% to 140% of the pulmonary valve annulus, and high-pressure balloons should be used, after obtaining an intermittent angiographic evaluation, using, for example, a Multi-track catheter that is positioned over the in situ guidewire in the RVOT. However, it is important to recognize that relief of the valvar stenosis may unmask a dynamic infundibular obstruction in some cases resulting in a persistent residual right ventricular outflow gradient (Fig. 17.5). This secondary area of obstruction can be documented by pressure recording during careful catheter withdrawal from the pulmonary artery to the RVOT or with simultaneous pressure recordings from a double-lumen catheter or from separate catheters in each of the two areas. Experience has shown that the infundibular obstruction is dynamic and that it will regress with time. This is particularly notable in adult patients undergoing balloon pulmonary valvuloplasty. Fawzy and colleagues reported an incidence of infundibular gradients in excess of 30 mm Hg in 46% of 93 adult patients undergoing balloon pulmonary valvuloplasty (75,76). All these patients were re-catheterized within 6 to 24 months, documenting a decrease in the mean infundibular gradient from 43 to 25 mm Hg. In less than 10% of patients, a dysplastic pulmonary valve is encountered, with thickened, redundant leaflets resembling a “cauliflower”; frequently supravalve stenosis may coexist. This condition is more common in some genetic conditions, such as Noonan’s syndrome. A higher-pressure balloon is usually required with a gradient reduction frequently less than what would be expected with nondysplastic valves. Within the recent multicenter experience from the C3PO registry reported by Holzer and colleagues, acute procedural success was 77%, with independent risk factors for procedural failure being the presence of a genetic syndrome, complex two-ventricle anatomy, presence
of supravalve pulmonary stenosis, a hemodynamic vulnerability score of 2 or more, as well as the need for balloon inflation above 8 atmospheres (24).
of supravalve pulmonary stenosis, a hemodynamic vulnerability score of 2 or more, as well as the need for balloon inflation above 8 atmospheres (24).
Procedure-related serious adverse events are rare. In a multicenter registry (C3PO) including 290 patients, the incidence of any adverse events was 9%, and high severity adverse events occurred in less than 3% (74). There was no incidence of procedure-related death, and independent predictors of higher level adverse events included among others age below 1 month, complex two-ventricle anatomy, two or more parameters of hemodynamic vulnerability, as well as operator experience of less than 10 years.
The long-term effects of the procedure have not yet been determined. However, studies so far have documented rates of restenosis between 5% and 11% within 10 years after the procedure (69,70). The risk of recurrence or restenosis may be greater in patients who present in infancy or with very dysplastic pulmonary valves, as well as those in whom an undersized balloon was used in the initial procedure. So far, reports have not provided evidence to suggest an increased risk of patients requiring pulmonary valve replacement because of pulmonary insufficiency, secondary to balloon pulmonary valvuloplasty. Dr. Charles Mullins pointed out that in the presence of otherwise normal heart and lungs, the regurgitant fraction is usually small and at low diastolic pressure, due to 80% to 85% of the ejection fraction having “diffused completely into the distal pulmonary capillary bed by the end of systole.” However, we have also learned that progressive right ventricular dilation secondary to pulmonary insufficiency should be considered a potential indication for pulmonary valve replacement.
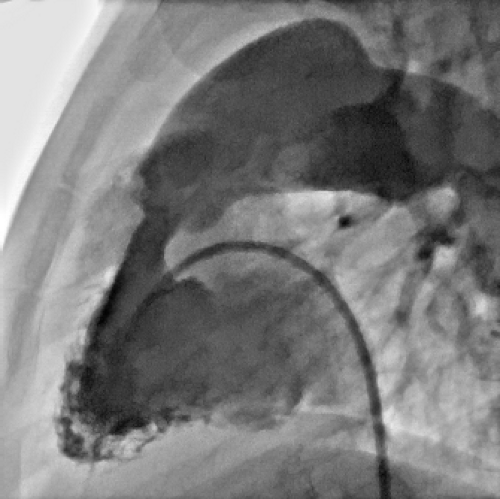
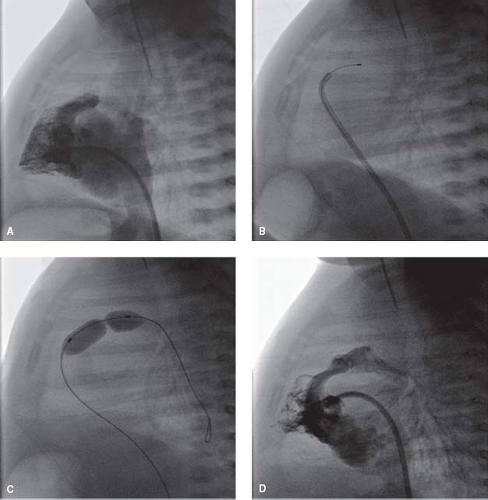
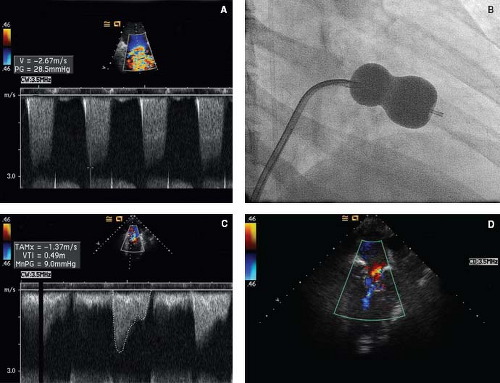
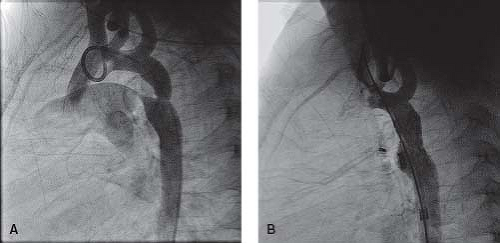
Perforation of the Atretic Pulmonary Valve
The diagnosis of pulmonary atresia with intact ventricular septum (PA/IVS) is usually made within the neonatal period. Pulmonary blood flow after birth is maintained through a patent arterial duct until a more definitive source of pulmonary blood supply can be established. An intra-atrial communication allows the systemic venous return to pass into the systemic circulation. The long-term prognosis of patients with PA/IVS can be extremely poor, especially when a diminutive right ventricle is combined with an RV-dependent coronary circulation with multiple coronary abnormalities. In this situation patients may require cardiac transplantation early in life. However, while patients with the diagnosis of PA/IVS and a single-ventricle pathway usually have a very poor long-term outcome, the outlook for those patients with a biventricular or a “one-and-a-half ventricle” circulation is much better.
Perforation of the atretic pulmonary valve plate has an important role to play within the available treatment modalities for these patients (61). Achieving antegrade pulmonary flow not only acutely decompresses the right ventricle, but more importantly serves as an incentive to facilitate further growth of an initially hypoplastic right ventricle. While a variety of sharp instruments as well as laser-guided techniques have been used to perforate the atretic pulmonary valve, these techniques are often poorly controlled and associated with a variably high risk of creating inadvertent injury to surrounding structures, often with disastrous results. Consequently, the use of RF energy was introduced into therapeutic cardiac catheterization in the early 1990s as an alternative to laser-guided perforation of the pulmonary valve plate (77).
The equipment presently most frequently used to achieve perforation of the atretic pulmonary valve with RF energy is the Nykanen RF perforation wire and the Baylis RF puncture generator (Both: Baylis Medical Corporation, Montreal, Quebec, Canada). Suitability for transcatheter RF perforation of the pulmonary valve plate is ascertained by 2-D echocardiography with minimal criteria in most cases being the presence of a tripartite right ventricle as well as a membranous atretic pulmonary valve with a well-formed infundibulum (78). Vascular access is routinely obtained via right femoral venous cannulation. A femoral arterial pressure monitoring line is placed. Initial hemodynamic evaluation includes measurement of right ventricular and systemic arterial pressures, followed by right ventricular angiography with 20-degree cranial angulation of the frontal tubes and standard lateral projection. This allows measurement of the pulmonary valve plate diameter and exclusion of RV-dependent coronary circulation. Further angiography is obtained in the left ventricle in the same projection. The combination of these two ventriculograms allows documentation of the relationship between the blind ending right ventricular infundibulum and main pulmonary artery. A 5-Fr Judkins 2.5 right coronary artery catheter is placed below the pulmonary valve plate within the RVOT using a Touhy Borst adapter to allow passage of the RF wire and simultaneous contrast injections. Once the RF wire and coaxial catheter are loaded, and accurate positioning is confirmed, RF energy is applied while maintaining a gentle push on the RF wire toward the valve membrane. In most patients a power setting of 5 W/sec for 2 seconds should be sufficient to perforate the pulmonary valve plate and it is important for the operator to be particularly suspicious of creating a false track if high-power settings are required. RF energy is discontinued once the wire has advanced through the valve plate. Appropriate position is confirmed using contrast injection through the Touhy Borst adapter. The coaxial catheter is then advanced over the RF wire into the main pulmonary artery and the RF wire is exchanged to a 0.014- or 0.018-inch coronary wire, which can be directed either to a position in a distal branch pulmonary artery or preferably through the PDA into descending aorta. Even though a wire position in the distal pulmonary arteries or preferably across the PDA needs to be established, this should not be attempted with the Nykanen RF wire, as it can easily cause perforation of the main pulmonary artery (61). The use of a coaxial catheter is a significant advantage when comparing the Baylis to the Osypka RF system that is still in widespread use elsewhere. A low-profile balloon valvuloplasty catheter, such as the Mini-Tyshak (NuMED, Hopkinton, NY), with a diameter of about 130% of the valve plate annulus is then advanced over the coronary wire and balloon dilation performed (Fig. 17.6). In cases where the balloon catheter cannot be advanced across the valve, sequential dilatation can be performed starting with a lower-profile 2.5-mm coronary balloon. Trackability can also be improved through arterial fixation or snaring of the coronary wire (79). Balloon valvuloplasty is followed by assessment of the pressure gradient across the pulmonary valve as well as the RV-to-systemic pressure ratio, and a final RV angiography is performed to document the result of the procedure.
A number of studies have reported on the outcome of RF perforation of the pulmonary valve and results have been summarized by Benson and colleagues (80,81,82,83,84,85). Most series are very small and include less than five patients. The overall procedural mortality is about 8% with incidence of procedural complications being about 15%.
It has been advocated that the combination of ductal stenting with RF perforation in one single procedure may reduce the number of transcatheter interventions and prevent prolonged postprocedural care. Approximately 50% of all neonates undergoing successful perforation and balloon valvuloplasty require additional pulmonary blood flow (86), even though it remains difficult to predict this for each individual patient. Some authors have suggested to use the tricuspid valve z-score (83), but this has not consistently been identified of being predictive of the need for pulmonary blood flow augmentation. Regardless, after failing to wean from PGE infusion after 5 to 7 days, one should proceed with either PDA stenting or surgical aortopulmonary shunt placement.
If performed appropriately, this technique allows up to 75% of suitable patients to ultimately sustain a biventricular or one-and-a-half ventricle circulation (61).
Transcatheter Treatment of Stenotic AV Valves
MV Dilation
When discussing balloon dilation of stenotic AV valves it is very important to distinguish between congenitally stenotic AV valves and acquired AV valve stenosis as a result of rheumatic fever. In many countries outside the US, rheumatic MV stenosis is still a common lesion in children. These acquired valve lesions with commissural fusion lend themselves naturally to a dilation procedure, which has been demonstrated to be effective in children. In contrast, the anatomy of congenital mitral stenosis (MS) is quite variable and is generally less favorable for balloon dilation, although the procedure has occasionally been effective for this lesion. The decision to proceed with balloon valvuloplasty of congenital MS should be based on a complete echocardiographic assessment of MV anatomy.
The MV is approached from the femoral vein using a transseptal approach into the left heart. A variety of techniques have been described which include predilating the atrial septum to allow passing a single large mitral dilation balloon, the passing of two balloons through two large transseptal sheaths, the passing of two balloons over separate guidewires without long sheaths and without the need for specific dilation of the septum, as well as a double-balloon technique using a Multi-track system over a single guidewire (NuMED, Hopkinton, NY) (87). The Inoue balloon (Toray Industries, Houston, TX) is a custom-made hourglass-shaped balloon that is available as a complete set, including sheath, guidewire, and mandrill. It specifically facilitates
balloon valvuloplasty using a single-balloon technique without the use of a guidewire in the left ventricle (Fig. 17.7). The Inoue balloon is available in three sizes, allowing balloon dilation diameters between 22 and 30 mm. Its use has demonstrated success in treating rheumatic MV stenosis (88).
balloon valvuloplasty using a single-balloon technique without the use of a guidewire in the left ventricle (Fig. 17.7). The Inoue balloon is available in three sizes, allowing balloon dilation diameters between 22 and 30 mm. Its use has demonstrated success in treating rheumatic MV stenosis (88).
When using a double-balloon technique, the left atrium is entered and one or two separate transseptal punctures are made. Exchange wires are manipulated from the right atrium through previously positioned catheters or long sheaths across the septum, across the MV, and into the left ventricle with the “transition” and floppy portions of the wires looped in the ventricle. It is important to remember that if a guidewire is to be used for balloon dilation, a balloon-tipped end-hole catheter should be initially directed from the left atrium across the MV to lessen the chance of crossing between chordal attachments. Once the wires are in place, either the two long sheath/dilator sets or the two separate “uncovered” balloons are passed over the wires into the left atrium. The balloons are advanced and positioned across the MV. The sheaths are withdrawn off the balloons and the balloons are simultaneously inflated. Again, longer balloons (5 to 6 cm in older children and adolescents) help to stabilize during inflation. The sum of the two balloon diameters equals the measured or estimated maximal normal MV diameter for a patient of that particular body size. The two balloons allow an adequate total balloon diameter for the much larger mitral annulus without coincident destruction of the entry veins or the atrial septum during balloon passage. As with any interventional catheterization, these patients require anticoagulation with heparin.
The initial success of transcatheter balloon dilation of congenital MV stenosis appears equal to that of surgical commissurotomy, depending on the MV apparatus. However, the total experience is limited, and the duration of the relief of the obstruction is unpredictable. McElhinney and colleagues recently described a series of 108 patients with congenital MS who underwent balloon mitral valvuloplasty (BMVP) or surgical intervention at a median age of 18 months (89). BMVP was effective in creating a reduction of the mean gradient by 38% while significant mitral regurgitation was identified as a result in 28% of procedures. Overall 5-year survival was 69% while patients at the later stages of the institutional experience had a 5-year survival of 87%. The early mortality was similar for balloon valvuloplasty and surgical mitral valvuloplasty, while the need for initial MV replacement was a significant predictor of worse acute and long-term outcomes. The 5-year survival free from failure of biventricular repair after balloon valvuloplasty was 75%. While the authors suggest that the initial procedure for congenital MS in patients with either typical congenital MS, or double orifice
MV should be balloon valvuloplasty, they concede that the surgical approach is more appropriate in patients with supravalve mitral ring and parachute MV.
MV should be balloon valvuloplasty, they concede that the surgical approach is more appropriate in patients with supravalve mitral ring and parachute MV.
While in general a parachute MV is not a contraindication to balloon valvuloplasty, the procedure is probably less likely to be effective when there is a single papillary muscle or severe shortening or virtual absence of the chordal apparatus (the “arcade-type” MV). Ultimately, it is difficult to predict which intervention is the more appropriate for congenital MS and most series report institutional preferences. As such, comparisons between surgical and percutaneous approach are biased at best.
Tricuspid Valve Dilation
Congenital tricuspid stenosis, as well as rheumatic, carcinoid, and rare other types of acquired tricuspid valve stenosis, occur less frequently than MS; but, as with MS, may be amenable to balloon valve dilation. Congenital forms of tricuspid valve stenosis are usually associated with other cardiac lesions and, similar to MV stenosis, are less amenable to balloon valvuloplasty than rheumatic tricuspid valve stenosis. The diagnosis of tricuspid stenosis is confirmed hemodynamically. As much information as possible about the valve anatomy and the exact location of the obstruction is obtained by echocardiography and angiography. The tricuspid valve is approached by passing one or two guidewires across the valve, either into the pulmonary artery or to the right ventricular apex. With the use of the calculated or estimated maximum tricuspid valve annulus diameter according to the patient’s body surface area, the balloon diameter or combined balloon diameters are chosen to equal this measurement (except in cases of annular hypoplasia). The dilation balloons are introduced through standard venous sheaths and over the wires. When the balloons are positioned across the stenotic valve, they are inflated simultaneously. Disappearance of the indentations or waists in the balloons at maximal inflation is sought. As with MV dilation, the use of the longer balloons facilitates appropriate positioning and maintaining positioning across the valve during dilation. A successful dilation should eliminate any transvalvar gradient. Experience with tricuspid valve dilation is limited; but, on the basis of even limited experience and minimal risk, this procedure is offered to the appropriate patients before considering surgery for tricuspid stenosis.
Transcatheter Management of Coarctation, Re-Coarctation, and Aortic Arch Obstructions
Dilation of peripheral arteries was the first therapeutic catheterization procedure and represents yet another area in which the vascular radiologist introduced the techniques. Dotter and Judkins (1) reported on the dilation of atherosclerotic peripheral arteries at the same time that Rashkind and colleagues were working on the atrial septostomy catheter.
The technique for dilation of vessel stenosis uses small, cylindrical, fixed maximal diameter dilating balloons passed over a spring guidewire, positioned across the area of stenosis, and inflated with relatively high pressures. This stretches or tears the area of stenosis up to the predetermined diameter of the balloon. This balloon technique is not only used to treat recurrent or native coarctation of the aorta, but similarly is used for branch pulmonary artery stenoses as well as central and peripheral venous stenoses. As many vessel dilation procedures are associated with immediate recoil and subsequent restenosis, the addition of balloon-expandable stents has further improved upon the available therapeutic interventional armamentarium and is frequently the treatment of choice in adult patients.
In 1979, Sos first experimented with balloon angioplasty of native coarctation in postmortem specimens (90) and this was followed by some fundamental work by Dr. James Lock in the early 80s. He performed balloon angioplasty on excised human coarctation segments as well as experimentally induced coarctation in lambs (91,92). He showed that balloon angioplasty achieved its therapeutic result by creating micro- or macroscopic intimal and medial tears over a variable distance in the vessel. These intimal injuries appeared to have healed completely on late pathologic examination, leaving the ballooned segment with a normal looking intima. The studies also documented that a balloon diameter of less than twice the size of the coarctation segment was unlikely to achieve a successful dilation, while diameters greater than three times appeared to carry a higher risk of deep and extensive tears.
Dr. Ronald Grifka’s and Dr. Charles E. Mullins’ group from Texas Children’s Hospital set the stage for early evaluation of endovascular stent therapy to treat coarctation (Fig. 17.8) (93). In animal experiments, they implanted Palmaz P308, 188 and 108 stents in the abdominal aorta and documented that stents can be safely redilated
after initial stent implantation. Dr. Andrew Redington added to this in 1993 by implanting a self-expandable stent into a 10-week-old girl who had previously had palliative surgery for HLHS (94). While one should generally avoid endovascular stent therapy in small infants, in some very rare circumstances the implantation of a low-profile stent in very sick infants may be justifiable, even though one has to accept that these low-profile stents are not expandable to adult size and therefore will eventually require surgical removal. In 1995, Suarez de Lezo reported the first large series of stent implantation to treat native and recurrent coarctation in humans using the Palmaz stent (95) and in 1999 Cheatham first reported on a new stent design, the CP stent, which is also available in a PTFE-covered variety (96). Holzer and colleagues at Nationwide Children’s Hospital reported a larger experience of stent therapy for complex aortic arch lesions, which further expanded the indications for transcatheter stent therapy with excellent results (Fig. 17.9) (97).
after initial stent implantation. Dr. Andrew Redington added to this in 1993 by implanting a self-expandable stent into a 10-week-old girl who had previously had palliative surgery for HLHS (94). While one should generally avoid endovascular stent therapy in small infants, in some very rare circumstances the implantation of a low-profile stent in very sick infants may be justifiable, even though one has to accept that these low-profile stents are not expandable to adult size and therefore will eventually require surgical removal. In 1995, Suarez de Lezo reported the first large series of stent implantation to treat native and recurrent coarctation in humans using the Palmaz stent (95) and in 1999 Cheatham first reported on a new stent design, the CP stent, which is also available in a PTFE-covered variety (96). Holzer and colleagues at Nationwide Children’s Hospital reported a larger experience of stent therapy for complex aortic arch lesions, which further expanded the indications for transcatheter stent therapy with excellent results (Fig. 17.9) (97).
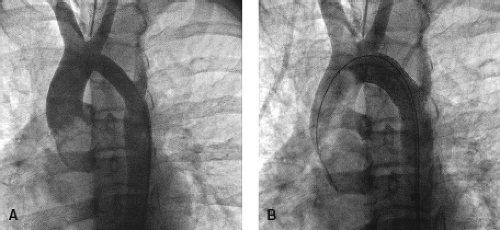 Figure 17.9 Complex arch stenting. A 15-year-old child with a history of coarctation repair (end-to-end) during infancy. Shone’s complex with transverse arch hypoplasia. Placement of a 36-mm Max LD stent in the transverse arch led to a gradient reduction from 22 mm Hg peak systolic to 2 mm Hg peak systolic. A: Aortogram before stent placement. B: Aortogram after stent placement.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|