Therapeutic Angiogenesis
G. Chad Hughes
Brian H. Annex
Atherosclerosis is the leading cause of morbidity and mortality in the Western world (1). The term therapeutic angiogenesis describes the field of cardiovascular medicine whereby new blood vessels are induced to grow to supply oxygen and nutrients to cardiac or skeletal muscle rendered ischemic as a result of progressive atherosclerosis (2). This chapter focuses on therapeutic angiogenesis as it applies to the treatment of patients with ischemic heart disease and peripheral arterial obstructive disease (PAD).
Scope of Clinical Problem
Coronary artery disease (CAD) continues to be the leading cause of mortality in the industrialized world, with more than 13 million Americans alive today with a history of angina pectoris, myocardial infarction, or both (3). Despite advances in pharmacologic therapies as well as catheter-based and surgical revascularization, significant numbers of these patients have diffuse CAD, small distal vessels, or other comorbidities that make them poor candidates for traditional methods of treatment. This number may represent as many as 12% of all patients with symptomatic CAD (4). As the average age of the population increases, the proportion of patients who are ineligible for traditional therapies will likely increase. Consequently, alternative means of improving blood flow to the heart such as therapeutic angiogenesis may take on a larger role in the treatment of CAD.
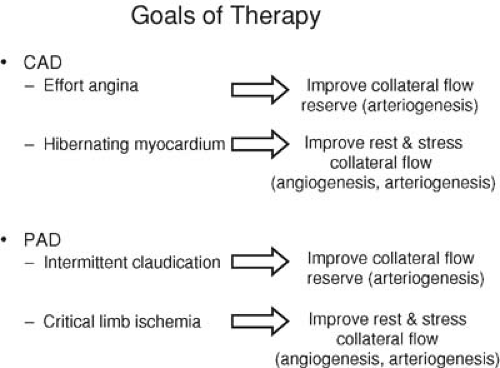
To date, clinical trials of therapeutic angiogenesis in CAD have generally been restricted to patients with refractory angina pectoris and so-called “end-stage” CAD (2). This term refers to patients with the persistence of severe anginal symptoms (Canadian Cardiovascular Society [CCS] class III and IV) despite maximal conventional antianginal combination therapy and coronary atherosclerosis not amenable to revascularization by percutaneous means or surgical bypass. Most of these “no-option” patients have multivessel CAD and have undergone one or more prior revascularization procedures (5). However, even though they typically have long-standing and diffuse disease, the patients selected for study of these alternative therapies generally have only mildly to, at worse, moderately impaired left ventricular function. Consequently, heart transplantation is not an option, and the goals of early trials have been targeted toward softer end points such as exercise time or quality of life measures. On the contrary, patients with large areas of prior myocardial infarction and its attendant necrosis and scar formation are typically excluded because these changes are considered not reversible with improvements in myocardial perfusion (6). Rather, patients should have ischemic yet viable myocardium as demonstrated by positron emission tomography, thallium or technetium (99mTc)-sestamibi scintigraphy, dobutamine echocardiography, or magnetic resonance imaging (MRI), because this situation is reversible with increases in myocardial blood flow (6).
The other major manifestation of atherosclerosis is PAD, which itself encompasses a spectrum of clinical syndromes with an incidence and prevalence nearly equal to that of CAD. Approximately 15% of adults who are more than 55 years old have detectable hemodynamic impairments attributed to PAD, and, similar to the incidence of CAD, the number of patients with PAD can be expected to increase as the population ages (7,8). The two major clinical presentations of PAD are intermittent claudication and critical limb ischemia. In patients with intermittent claudication, arterial occlusive disease is manifested by insufficient blood flow during exercise, whereas in critical limb ischemia, blood flow is inadequate to meet the demands of the limb even at rest. Consequently, the goals of therapy for claudication are quite different from those of critical limb ischemia.
Standard therapy for PAD manifest as intermittent claudication includes atherosclerotic risk factor modification, smoking cessation, exercise, and pharmacologic therapy. Surgical revascularization, although effective, is generally not indicated except in lifestyle-limiting, medically refractory claudication. Percutaneous therapy is another option, although long-term results are poor except in proximal aortoiliac disease (9). For critical limb ischemia presenting as rest pain or tissue loss, mechanical revascularization with surgery or percutaneous intervention is the treatment of choice (9). However, these patients typically have significant comorbidities as well as diffuse, distal atherosclerosis that make mechanical revascularization both higher risk in the short term and less successful in the long term as a result of graft loss from small target vessels and poor runoff. Consequently, a search for new and more efficacious treatment options for PAD, including therapeutic angiogenesis, is ongoing (Fig. 103.1).
Postnatal Revascularization via Blood Vessel Growth
Neovascularization in ischemic adult cardiac and skeletal muscle is now recognized to result from the processes of angiogenesis, arteriogenesis, and vasculogenesis (Fig. 103.2). Angiogenesis refers to the sprouting of new capillaries lacking a developed tunica media from preexisting ones (10). In the adult, this process is mainly caused by hypoxia and is mediated via activation of hypoxia-inducible factor (HIF-1α), which serves to increase transcription of vascular endothelial growth factor (VEGF) and its receptors and to stabilize VEGF mRNA (11).
Arteriogenesis describes the type of vascular growth responsible for the production of vessels with a fully developed tunica media and capable of carrying significant blood flow as well as being visualized with angiography (10,11,12). This process may involve the maturation of preexisting collateral vessels or may reflect do novo formation of mature vessels (10). Preexisting coronary collateral vessels have been demonstrated even in patients with angiographically normal coronary arteries (13). These collateral vessels may be important for the process of arteriogenesis. Unlike angiogenesis, ischemia is not a prerequisite for arteriogenesis (14). Rather, primary arteriogenic stimuli include shear stress and inflammation in which an invasion of monocytes and other white blood cells leads to the production of growth factors such as the fibroblast growth factors (FGFs) and tumor necrosis factor-α (TNF-α) with subsequent vascular growth (15,16,17). Following arterial occlusion, monocytes/macrophages, in particular, accumulate in the tissue surrounding collateral vessels via ICAM-1/Mac-1 dependent mechanisms (18). Moreover, a recent study in the rabbit hind limb model clearly demonstrated that monocytes, and not granulocytes or T lymphocytes, are the key cellular mediators of arteriogenesis (17).
Vasculogenesis (19,20) describes the in situ formation of blood vessels from endothelial progenitor cells (EPCs) termed angioblasts (12,21). Angioblasts are CD34+ stem cells recruited from the bone marrow that are thought to migrate and fuse with other EPCs and capillaries to form a primitive network of vessels known as the primary capillary plexus. After this primary capillary plexus is formed, it is remodeled by sprouting and branching via the process of angiogenesis. However, some investigators have questioned the origin of these EPCs as well as whether EPCs truly incorporate into vessel walls in areas of vascular growth (22,23,24,25). Schmeisser et al. demonstrated that CD34– monocytes may develop an endothelial phenotype and even form tube-like structures in vitro, a finding suggesting a potential role for non–stem cell monocytes in vasculogenesis (22). Further, Rehman et al. (23) found that most so-called EPCs are actually derived from the monocyte/macrophage lineage, with only a small population of true CD34+ stem cells/progenitor cells that originate from hematopoietic stem cells/angioblasts. These monocyte/macrophage–derived “circulating angiogenic cells” secrete multiple angiogenic growth factors that likely mediate their angiogenic effects. Finally, Ziegelhoeffer (24) published a study suggesting that, in the adult organism, bone marrow–derived stem cells do not promote vascular growth by incorporating into vessel walls but rather via paracrine effects from the production of multiple angiogenic cytokines. Regardless of the true mechanism, however, vasculogenesis appears to be a real phenomenon in the postnatal organism, and vasculogenesis, angiogenesis, and arteriogenesis all potentially contribute to neovascularization in adult cardiac and skeletal muscle (12,26), although some authors (16,27) have suggested that arteriogenesis is necessary for significant improvements in blood flow.
Therapeutic Angiogenesis
The endothelium in adults normally exists in a quiescent state. Only when provoked by stress or pathologic conditions does the vascular bed expand via the processes of vasculogenesis, angiogenesis, and arteriogenesis (26




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree