The Replacement of the Aortic Valve with the Pulmonary Autograft
Mani Arsalan
J. Michael DiMaio
William H. Ryan
The Ross Procedure (RP) is indicated for the replacement of the aortic valve in patients with severe, symptomatic aortic stenosis or aortic insufficiency (AI) if they are in good health and their life expectancy is not limited by age or medical conditions unrelated to their aortic valve disease. The RP is commonly performed in young and middle-aged adults who suffer from congenital or bicuspid aortic valve disease and/or endocarditis of the aortic valve. It is used occasionally in older, high-performing adults and in any patient where anticoagulation is dangerous or carries unacceptable lifestyle changes. The autograft aortic valve performs well during exercise with gradients in the single digits; therefore, the RP is an excellent aortic valve replacement (AVR) technique for athletes.
Although use of the procedure in congenital or dysplastic valves is widely accepted, the indication for RP in patients with bicuspid valve disease is more controversial. The associated aortopathy in 40% of patients with bicuspid aortic valves implies neural crest abnormalities as the initial etiology, which can lead to dilation of the autograft root postoperatively. During the last several years, it has become apparent that patients with aortic stenosis and a normal aortic root and ascending aorta can remain free from reoperation for many years. On the other hand, patients with aortic dilation and insufficiency fare less well after the RP. Nevertheless, in the author’s experience, with the proper operative interventions, these patients remain free from intervention for up to 15 years. As surgeons gain experience with both bicuspid aortic valve reimplantation and valve-in-valve technology to treat insufficiency, however, the role of the autograft AVR in bicuspid aortic valve disease will undoubtedly change in the future.
The use of a pulmonary autograft necessitates cadaveric pulmonary root replacement, which makes the RP a two-valve operation to correct single-valve pathology. Initially, early reoperation for pulmonary homograft stenosis occurred in up to 20% of patients and led to reoperation in the face of a normally functioning autograft. With the use of oversized, decellularized pulmonary homografts, early stenosis is now rare. The use of male donors, in our experience, has partly facilitated this decrease. The pulmonary valve replacement now occurs at the time of autograft replacement or reimplantation.
Etiologic contraindications for the RP include patients who suffer from rheumatic or autoimmune aortic valve disease. These patients often have some involvement of their pulmonary valve as well and, therefore, should not undergo the RP. Likewise, patients who have undergone chest radiation are not candidates. While the appearance of the pulmonary valve leaflets may be benign on inspection in patients with prior radiation exposure, adaptation to the systemic circulation unmasks pathologic changes and leads to leaflet scarring and retraction.
Morphologic contraindications for the RP include pulmonary leaflet and artery abnormalities and coronary contraindications. Pulmonary valves with large pulmonary leaflet fenestrations or multiple fenestrations within opposing leaflets should not be used. Small fenestrations with an opposing, normal leaflet are acceptable autograft candidates. Very thin main pulmonary arteries or sinuses where the pulmonary artery (PA) catheter is visible are occasionally encountered and should not be incised. An aortic annulus more than 4 mm larger than the pulmonary annulus is a relative contraindication and requires an aortic annuloplasty. Of note, aortic annuloplasty has been associated with earlier autograft reoperation.
An appropriate preoperative work-up will disclose anomalous coronary arteries, which can make the RP problematic. While an anomalous right coronary artery (RCA) off the left coronary sinus is not a contraindication, the location of reimplantation must be chosen with care. Other coronary anomalies such as the left main (LM) coronary artery arising from the RCA or an aberrant circumflex are contraindications. A very short LM coronary artery—though not a contraindication—makes reimplantation more technically demanding.
Before undergoing the RP, a patient must be given a clear explanation of all options available with adequate teaching materials and time for reflection and follow-up discussion. The surgeon’s experience and the expected outcome should be discussed in addition to the importance of compliance with daily medications, annual follow-up echocardiography, and occasional CT scanning. Medical management of hypertension, which is often present as a comorbidity, should include routine use of a beta-blocker and an ACE inhibitor, if needed. In patients with congestive heart failure (CHF) symptoms or poor left ventricular (LV) function, carvedilol as well as an ACE inhibitor should be titrated using sequential echocardiography to optimize LV performance prior to the procedure.
Preoperative studies include both transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE), computed tomography angiogram (CTA) of the thoracic aorta, and evaluation of the coronary arteries. TTE evaluation of aortic valve disease confirms velocities, gradients, and chamber sizes, while TEE determines aortic and pulmonary annulus compatibility and the presence or absence of pulmonary insufficiency. CTA gives an accurate measurement of the aortic dimensions from annulus to diaphragm and, if done as a coronary CTA, provides excellent anatomic coronary evaluation. In older patients or patients in whom coronary atherosclerosis is a concern, coronary angiography is indicated. If possible, patients taking anticoagulation other than heparin or aspirin should hold this medication preoperatively for the appropriate time determined by the drug’s half-life.
Incision and Cannulation
The procedure is performed through a standard median sternotomy. The pericardium is opened midline, and a 1- to 2-mm strip is harvested from each edge for use in anastomotic hemostasis. Pericardial stay sutures are placed. Aortic cannulation is guided by the pre-op CTA and should be high enough to allow replacement of any dilated aorta.
In rare cases when a hemiarch is to be performed, axillary cannulation is recommended. Double venous cannulation with caval tapes is often used, but single venous cannulation with an ice-filled 4 × 4 gauze sponge placed on the anterior surface of the right ventricle (RV) works well to prevent venous air locks during the procedure. Both retrograde and antegrade cardioplegia given regularly with topical RV cooling provide excellent myocardial protection. Cardiopulmonary bypass is instituted and cooling to 30°C is begun. Once the heart is empty, the cross-clamp is placed at the pericardial reflection, and cardioplegic arrest is induced.
In rare cases when a hemiarch is to be performed, axillary cannulation is recommended. Double venous cannulation with caval tapes is often used, but single venous cannulation with an ice-filled 4 × 4 gauze sponge placed on the anterior surface of the right ventricle (RV) works well to prevent venous air locks during the procedure. Both retrograde and antegrade cardioplegia given regularly with topical RV cooling provide excellent myocardial protection. Cardiopulmonary bypass is instituted and cooling to 30°C is begun. Once the heart is empty, the cross-clamp is placed at the pericardial reflection, and cardioplegic arrest is induced.
Pulmonary Autograft Evaluation and Excision
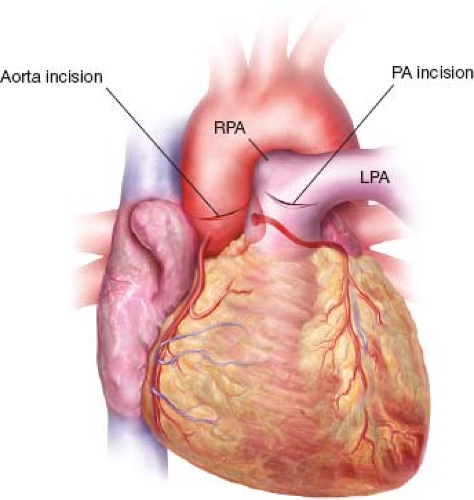
The pulmonary autograft is evaluated first. The main PA is opened anteriorly at its junction with the right main PA, and the incision is carried toward the left main PA to expose the pulmonary valve (Fig. 8.1). All three leaflets are evaluated carefully, and, if they are deemed suitable, the main PA is transected below the bifurcation. Care to avoid incorporating any proximal right PA will aid in reconstructing the pulmonary outflow tract. The pulmonary homograft is thawed at this time. The aorta is then transected parallel to the sinotubular junction (STJ), and a small silk suture is placed adjacent to the LM coronary artery on the ascending aorta (Fig. 8.1). Autograft excision is done with the LM coronary artery visualized at all times (Fig. 8.2). Dissection begins posterior to the PA, leaving its adventitia in place. The LM coronary artery falls below the plane of dissection, and a clear rim of ventricular muscle is visualized (Fig. 8.3). Dissection anteriorly between the PA and aorta on the aortic side of the fibrous body mobilizes the autograft posteriorly (Fig. 8.4). Under direct visualization through the PA, a right-angle clamp is placed 2 to 3 mm below the nadir of the anterior pulmonary leaflet and punched through to the right ventricular outflow tract (RVOT) in order to mark the start of the autograft excision line (Fig. 8.5). This opening is enlarged parallel to the RVOT muscle fibers until the annulus of the autograft is visualized. The autograft is then excised 3 mm below the annulus in a circumferential manner (Fig. 8.6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree