The Natural History and Noninvasive Treatment of Lower-extremity Arterial Occlusive Disease
Burress M. Welborn III
Franklin S. Yau
James M. Seeger
Epidemiology
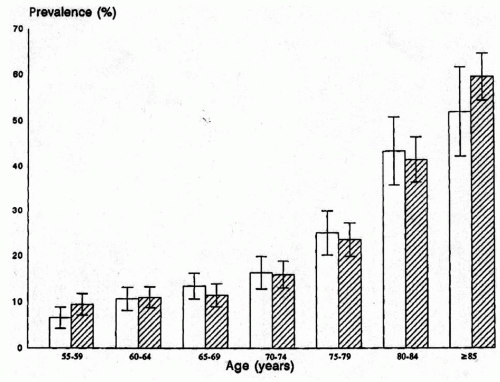
Peripheral arterial occlusive disease is a common disease of the elderly. Although the pathologic changes that are precursors of atherosclerosis can be identified in children, clinically significant disease is rare before the sixth decade. Predictably, the incidence of peripheral arterial occlusive disease and intermittent claudication continues to increase with age among the elderly (Fig. 43-1). Approximately 20% of the elderly population has evidence of atherosclerotic lower-extremity arterial occlusive disease, but the incidence of symptoms associated with these lesions is significantly less (<5%). Recent studies have also suggested that lower-extremity musculoskeletal symptoms and functional limitations are more common in patients with documented peripheral arterial occlusive disease, even when the classical symptoms of claudication are absent. The majority of patients with symptomatic occlusive disease will have only mild symptoms of claudication, and the likelihood of patients with claudication progressing to limb-threatening ischemia over 10 years is generally low, although it is increased among patients with more severe claudication (i.e., shorter distances) and those with significant risk factors, such as diabetes mellitus. Thus, peripheral atherosclerosis is a common finding with a fairly benign natural history and small risk of limb loss. Only a small portion of patients with clinically significant peripheral arterial occlusive disease will require intervention. However, detection of peripheral arterial occlusive disease is critical because it is a marker for atherosclerosis in the other vascular beds and a significant risk factor for stroke and cardiovascular death.
Risk Factors
The risk factors for peripheral arterial occlusive disease have been well defined and include age, smoking, hypertension, hypercholesterolemia, family history, and the inflammatory mediators. Increasing age is an independent risk factor, and older patients generally present with lower ankle-brachial indices (ABIs) at the time of diagnosis. Both current and former smokers are at an increased risk of developing peripheral arterial occlusive disease, with former smokers having only a mild increased risk and active smokers having a relative risk more than double that associated with the former smokers. Indeed, it has been estimated that smoking may be the responsible mechanism in up to 50% of all cases. Not surprisingly, the age patients begin to smoke has also been identified as a risk factor, with greater risk associated with those who start at an earlier age.
The risk for developing peripheral arterial occlusive disease associated with hypertension and diabetes mellitus is comparable to that with smoking. The risk associated with hypertension has been shown to increase with systolic hypertension. Importantly, diabetes is also an independent predictor for progression to limb-threatening ischemia. The risk associated with diet-controlled diabetes is less than that associated with diabetes requiring either oral hypoglycemic agents or insulin therapy. The risks associated with hypercholesterolemia are less than those associated with smoking, hypertension, and diabetes. Furthermore, the breakdown of the lipoprotein profiles may be more significant than the total cholesterol levels; higher highdensity lipoprotein (HDL) levels lower the risk of peripheral arterial occlusive disease, while elevated low-density lipoprotein (LDL) levels increase the risk.
There also appears to be a genetic risk that contributes to the development of peripheral arterial occlusive disease. There is a subset of young men younger than 55 who present with a particularly aggressive form of the disease. Although these individuals are frequently heavy smokers, their asymptomatic, first-degree relatives often have a higher incidence of occult peripheral arterial occlusive disease compared to both the smoking and nonsmoking general population. This observation indirectly supports the hypothesis that a genetic predisposition plays a role in the development of the disease process both in this subset and the population as a whole. Unfortunately, the specific genetic factors that contribute are not well described. However, there is significant evidence that atherosclerosis is an inflammatory disease, and polymorphisms in the genes associated with the inflammatory response may contribute to the risk profile.
Elevated serum levels of fibrinogen and C reactive protein are also associated with the development of peripheral arterial occlusive disease. Fibrinogen and C reactive protein are acute phase reactants, secreted during states of inflammation. Interleukin (IL)-6 is the primary signal for these proteins, and the serum levels of IL-6 have been correlated with an increased risk of coronary artery disease. The role that IL-6 plays in the development of peripheral arterial occlusive disease is less clear. Soluble receptors for proinflammatory cytokines TNFα and IL-1β have also been found to be elevated in the patients with peripheral arterial occlusive disease, indicating an overproduction of
these proinflammatory cytokines. Homocysteine and lipoprotein also increase the risk of peripheral arterial occlusive disease, although the correlation is not as strong as that for the other inflammatory proteins.
these proinflammatory cytokines. Homocysteine and lipoprotein also increase the risk of peripheral arterial occlusive disease, although the correlation is not as strong as that for the other inflammatory proteins.
Management Principles
The management of symptomatic peripheral arterial occlusive disease is palliative, and the primary objectives are to reduce the symptoms and prevent limb loss. There are no medical or noninvasive treatments that reverse the peripheral occlusive lesions, although the statins (HMG-CoA reductases) may reverse the coronary atherosclerotic lesions. The noninvasive therapies are designed to treat the symptoms and/or attempt to halt the clinical progression of the disease. The advent of the endovascular therapies has allowed vascular specialists to treat some patients with symptomatic peripheral occlusive disease in a less invasive manner, thus potentially expanding the percentage of patients in whom intervention is indicated. However, most patients who will benefit solely from endovascular therapies have a low disease burden that can often be treated with equal success using medical therapies alone. Only patients with significant symptoms and those with a significant risk of limb loss as predicted by the severity of their disease process should be considered for invasive therapy. Furthermore, the decision to operate should be made only after careful consideration of the risk/benefit ratio for the patient. Essentially all patients with peripheral arterial occlusive disease have significant, concurrent coronary artery disease that increases their risk for surgical intervention and limits their life expectancy. Indeed, the peri-operative mortality rate after open peripheral revascularization ranges from 1% to 5%, while the incidence of wound infection, bleeding complications, and amputation is also significant. Because of these concerns, a thorough understanding of the risks of invasive therapy and the natural history of the disease process is imperative.
Peripheral arterial occlusive disease involves the arterial tree proximal to the ankle in virtually all cases, including patients with diabetes mellitus. It is a common misconception that diabetics suffer from “small vessel disease” of the foot that precludes arterial reconstruction. While diabetics do have a high rate of infrapopliteal arterial occlusive disease, they do not suffer from microvascular or arteriolar disease of the foot. Furthermore, virtually all patients with diabetes mellitus and ischemic ulcerations have suitable anatomy for surgical revascularization.
Diagnosis and Vascular Laboratory Studies
The diagnosis of peripheral arterial occlusive disease is usually straightforward and based upon the history, the physical examination, and the noninvasive vascular laboratory studies. These components are also used to determine the severity of the disease process that helps predict its natural history.
Claudication is simply defined as pain in the major muscle groups of the lower extremity with exercise. It is commonly described as a “cramping sensation” or “Charlie horse” in the calves, but it can occur in the thigh and buttocks. Less commonly, patients complain of leg heaviness or state that their legs “go dead” after ambulating; falling associated with these symptoms is not uncommon. Because of the broad spectrum of symptoms, other pathologic processes, particularly lumbar sacral degenerative osteopathy with cord or nerve root compression, can masquerade as claudication. The symptoms associated with claudication occur at a reproducible distance and resolve completely with rest (<10 to 15 min). Any type of ambulation that requires increased energy expenditure, such as climbing up stairs, walking on an incline, or walking on uneven surfaces, will reduce the distance required to elicit symptoms. The distribution of the symptoms also corresponds to the level of disease, with the muscle group affected usually one anatomic level below the occlusive disease (i.e., calf claudication is associated most commonly with superficial femoral artery disease, while claudication in the thigh and buttocks indicates more proximal aortoiliac occlusive disease). However, calf claudication is the most common symptom in patients with aortoiliac disease.
Ischemic rest pain is associated with more severe occlusive disease and further hemodynamic compromise. It occurs when the perfusion is inadequate to meet the metabolic needs of the tissue and affects the most distal aspect of the arterial tree, the forefoot. Patients commonly complain of pain across the metatarsal heads (i.e., metatarsalgia), and this pain often includes the toes. The pain may occur only with elevation and frequently awakes patients from sleep. Indeed, it is important to ask patients about their sleeping habits while eliciting the history of present illness during their evaluation. Patients commonly attempt to augment their distal perfusion with positional changes. The most common maneuver is to hang the foot over the edge of the bed during sleep, but limited ambulation may also serve to relieve the pain. These maneuvers increase blood flow to the foot due to the forces of gravity and augmentation of cardiac output (ambulation). Patients with peripheral neuropathy can present with pain similar to ischemic rest pain, but it can usually be differentiated based on its characteristics and relationship to positioning. The pain related to peripheral neuropathy is commonly described as a “tingling or burning” sensation that is continuous, not related to
positioning, improved with elevation, located in a sock-like distribution, and bilateral. Often patients with neuropathy will complain of a foreign body sensation when walking, described as having “rocks in their shoes”. The ischemic rest pain can progress to the point that it is refractory to positional changes (i.e., dependency), and it can be difficult to control with pain medications.
positioning, improved with elevation, located in a sock-like distribution, and bilateral. Often patients with neuropathy will complain of a foreign body sensation when walking, described as having “rocks in their shoes”. The ischemic rest pain can progress to the point that it is refractory to positional changes (i.e., dependency), and it can be difficult to control with pain medications.
Further progression of the ischemic rest pain can result in tissue loss, although some patients present with tissue loss without antecedent rest pain, typically occurring following injury. The tissue loss can range from a shallow ulcer to extensive gangrene of the toes/forefoot. Ischemic ulcers are typically painful, involve the foot/toes, and defined as nonhealing if they persist for 4 to 6 weeks despite appropriate wound care. Gangrene is the most extreme form of ischemic tissue loss, and the presentation ranges from dry gangrene to wet gangrene to florid foot sepsis. Notably, soft tissue infections of the foot can be a life-threatening emergency that requires aggressive debridement and control of the sepsis prior to any attempt at revascularization.
The physical examination helps to confirm the diagnosis and to determine the anatomic level of the occlusive disease. Patients with aortoiliac disease will have absent or diminished femoral pulses, while disease of the superficial femoral artery is characterized by normal femoral pulses and absent popliteal pulses. Palpable femoral and popliteal pulses with absent pedal pulses suggest isolated infrapopliteal disease. Patients with mild claudication may have palpable pedal pulses at rest that become nondetectable after exercise. Chronic lower-extremity ischemia is associated with a variety of other adaptive changes, including hair loss, hypertrophy of the toenails, dry/scaling skin, muscular atrophy, and dependent rubor (ischemiainduced dermal vasodilation with dermal pooling). In patients with dependent rubor, the foot/calf appears red or purple, and this color change can be confused with cellulitis. Elevating the leg results in the loss of the dependent rubor (i.e., elevation pallor) and can help differentiate chronic limb ischemia from cellulitis. In patients with pigmented skin, dependent rubor is not seen, but chronic ischemia should be suspected when hyperpigmentation is present along with the other signs of ischemia.
Noninvasive vascular testing is critical for the diagnosis of peripheral arterial occlusive disease and serves to validate the patient history and physical examination. Indeed, the history and physical exam are associated with a false positive rate of up to 44% and a false negative rate of 19% among patients with significant peripheral occlusive disease. The primary noninvasive vascular study is the resting ABI. This is a simple test that quantifies the degree of hemodynamic compromise present in each lower limb, and, thereby, provides insight into the expected natural history of the disease process. The relationship between clinical symptoms and the ABIs is shown in Figure 43-2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree