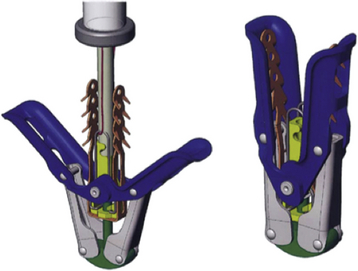
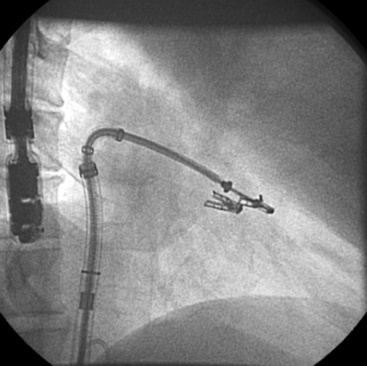
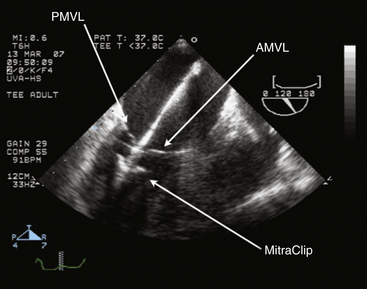
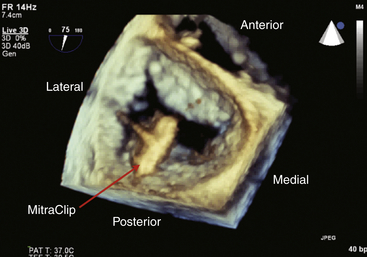
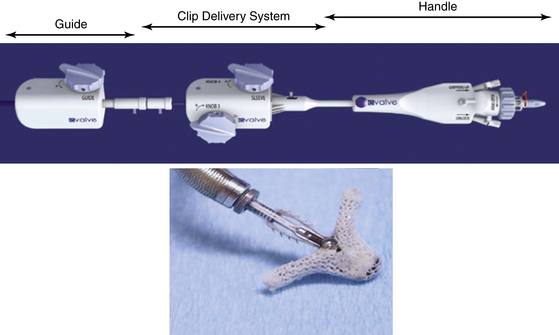
Chapter 11 • Percutaneous transcatheter therapies for mitral regurgitation (MR) have found a role for patients at high operative risk with both degenerative and functional pathologies. • The MitraClip (Abbott Vascular Structural Heart, Menlo Park, Calif.) therapy utilizes a catheter-based system to deliver a clip-type implant to provide apposition between anterior and posterior mitral leaflets. • Careful patient selection with a thoughtful eye to appropriate mitral pathology remains paramount for success with the MitraClip. • Technical success is dependent on skill with echocardiographic imaging, with three-dimensional transesophageal echocardiography (TEE) being particularly valuable. MR from nonrheumatic etiology may be the result of either degenerative valve disease, such as prolapse, chordal rupture, or myxomatous degeneration, or of functional etiologies such as ischemic cardiomyopathy and secondary MR. Additionally, as surgical or transcatheter therapies focus on functional etiologies of MR, the knowledge base of how the disease of the ventricle affects the mitral apparatus will increase. Significant MR (3+ or 4+) occurs in 0.5% of the population,1 with approximately 250,000 new cases annually in the United States. For patients with significant organic MR, medical therapy has been shown to be ineffective in treating the underlying pathophysiology and is unable to retard disease progression. Surgical repair or replacement is the standard of care, because it is efficacious and low risk.2 However, it has been estimated that only about 20% of patients with significant MR undergo surgery. Therefore there is a significant unmet therapeutic need for patients with mitral valve regurgitation, and particularly for patients at high surgical risk, a significant opportunity for novel catheter-based therapies. This novel percutaneous approach was the first to achieve success in prehuman and clinical trials for treatment of patients with nonrheumatic MR. The concept of grasping a leaflet with a clip mechanism on the regurgitant portions of the mitral valve became the hallmark of the final design iteration of the MitraClip (Figure 11–1).3,4 The MitraClip is introduced from a percutaneous, transvenous, transseptal approach to the mitral valve (Figure 11–2). The traditional imaging modality in the catheterization lab of fluoroscopy is of limited utility in this procedure, because it cannot visualize the mitral leaflets. Therefore the procedure is guided by simultaneous transesophageal imaging, ideally using both two- and three-dimensional echocardiography (Figures 11–3 and 11–4). Combined with the reality that the MitraClip delivery system is manipulated by a mechanical-based device, it is more important that the valve interventionalist be skilled in transesophageal imaging than in the standard catheter and wire skills. Figure 11–1 A schematic view of the MitraClip, used for percutaneous repair of MR, is shown. The blue arms are mechanically hinged and allow the operator to change the angle between them. These arms are designed to be pulled up from the ventricular side of the mitral leaflets to bring them together. The reddish spiked frictional elements may be lowered onto the atrial side of the leaflets, thereby sandwiching each leaflet between the blue arm and the red frictional element. (Image provided courtesy of Abbott Vascular Structural Heart, Menlo Park, Calif.) Figure 11–2 Fluoroscopic image demonstrates the transvenous, transseptal approach to introduce the MitraClip to the mitral valve. Figure 11–3 Two-dimensional TEE image of the MitraClip and its orientation to the anterior (AMVL) and posterior mitral valve leaflets (PMVL) is shown. This three-chamber view of the mitral valve is used to orient the MitraClip in an anteroposterior direction on the valve. Figure 11–4 Three-dimensional TEE image of the MitraClip and its orientation to the mitral valve is shown. The arms of the MitraClip are oriented perpendicular to the line of coaptation of the valve and over the point of pathology. To maintain operator orientation, the atrial septum is on the right side of the screen, with the aortic valve at approximately the 12 o’clock position. When the MitraClip is placed on the central portions of the anterior and posterior leaflets, it acts to anchor prolapsing or flail segments, as well as coapt tethered leaflets so that it reduces the time and force required to close the valve. By decreasing MR, the left ventricular (LV) volumes are in turn reduced, leading to beneficial left ventricular remodeling.5 Anatomically, the MitraClip creates a tissue bridge between the two leaflets that limits dilation of the mitral annulus in the septal–lateral dimension, which supports the durability of this repair.4 This MitraClip approach to percutaneous mitral valve repair was evaluated in the EVEREST trials. EVEREST I was the safety and feasibility registry and enrolled 55 patients in a nonrandomized clinical trial.6 EVEREST II was the pivotal clinical trial in which 279 patients with 3+ or 4+ MR were randomized to either MitraClip therapy or standard surgical therapy (either mitral valve repair or replacement).7,8 It is important to note that this involved patients at low and moderate risk, in contrast to the EVEREST High Risk Registry, which enrolled 79 patients who were nonoperative candidates to the MitraClip therapy. In the early nonrandomized experience with the MitraClip, procedural success (reduction of MR to 2+ or less) was achieved in 74%, and 66% were free from death, mitral valve surgery, or MR greater than 2+ at 12 months.8 These outcomes were similar for those with either degenerative (79%) or functional (21%) etiology of MR. It is also important to note that this represents the initial experience with this novel technology that has a steep learning curve. Compared with patients from either the Society of Thoracic Surgery database9 or those undergoing first time elective mitral valve surgery,10 those enrolling in the EVEREST II trial were significantly older with more comorbidities. This likely is because of the preference for the initial experience with novel percutaneous therapies to be reserved for patients who are less ideal surgical candidates. This randomized trial was designed to have a primary safety endpoint powered for a superiority hypothesis—for major adverse events at 30 days. The primary effectiveness endpoint was powered for a noninferiority hypothesis—for the 12-month composite of freedom from death, mitral valve surgery, reoperation, or MR greater than 2+. Additional endpoints included echocardiographic-assessed MR severity, left ventricular function, New York Heart Association (NYHA) functional class, and quality of life indices. From the primary safety endpoint, with an intention to treat statistical analysis, those randomized to the MitraClip arm had a major adverse event rate of 15% at 30 days, versus those in the surgical arm who had an event rate of 48% (psup < 0.0001).7 Further detailed analyses of the major adverse events such as death, stroke, reoperation, or emergent surgery demonstrated no such events occurring in patients receiving the MitraClip alone, but adverse events occurred in those crossing over to surgical therapy after unsuccessful MitraClip therapy. Therefore in a per-protocol analysis, which evaluates just the performance of the device alone rather than a strategy approach, the major adverse event rate was under 10% at 30 days. From the primary effectiveness endpoint, in terms of the clinical composite of freedom from death, mitral valve surgery/reoperation, or MR greater than 2+ at 12 months with an intention to treat analysis, the MitraClip arm had a success rate of 55% versus the surgical arm, which had a success rate of 73% (pNI < 0.0012). At 24 months, the primary effectiveness rate dropped in both groups to 52% and 66%.7 Further analysis of the events driving the composite endpoint demonstrates that there was no significant difference between the MitraClip arm and the surgical control arm with respect to death or freedom from severe MR, but that the difference in composite endpoints was the result of a difference in subsequent surgery for mitral valve dysfunction (p < 0.001). MR reduction to the goal and maintenance at 12 and 24 months of 2+ or less was achieved in 81% of the patients receiving the MitraClip, versus 97% of patients who had surgery, although this was achieved by mitral valve replacement in 12% of surgical patients. Despite this difference in echocardiographic-measured MR reduction, there were similar reductions in left ventricular dimensions and volumes achieved at up to 24 months. Interestingly, the left ventricular volumes continued to remodel beneficially between 1 and 2 years. Additionally and paradoxically, despite the differences in apparent MR reduction, there were a greater percentage of patients from the MitraClip arm who were asymptomatic or minimally symptomatic at 24 months (98%), versus those in the surgical arm (88%). Some of these apparent inconsistencies may be because of the difficulties in quantifying the degree of residual MR after a MitraClip has been placed in the center of the valve. Data presented showed that whereas at 30 days the predicted mortality for the group was 18%, the actual mortality was under 8%, with 76% surviving at 1 year and 79% of the survivors in New York Heart Association symptom class I or II.11 In a nonrandomized comparison to similar high-risk patients treated medically, there was a survival advantage at 1 year, with 76% alive in the MitraClip group, versus 55% in the medically treated group (p = 0.037). This data gives support to the concept that particularly for those patients who are not candidates for surgery, novel percutaneous options are attractive. Not all patients who have undergone percutaneous mitral valve repair with the MitraClip will have adequate reduction of MR or perfect durability of that repair. Clearly the time and energy spent up front with appropriate patient selection will pay dividends in terms of outcomes. However, for those patients who have inadequate or nonsustained MR reduction after MitraClip implantation, subsequent surgical repair has been performed. The rate of surgical repair of MR after MitraClip implantation was 89% (n = 20), which compares favorably to the repair rate in those randomized to the surgical control arm of EVEREST II (86%, n = 69), or as found in the 2007 Society of Thoracic Surgery database (59%). Anterior or bileaflet disease was an independent predictor of mitral valve replacement after MitraClip therapy, but age, etiology, or surgeon experience was not. Additionally, successful mitral valve surgery has been performed up to 5 years after MitraClip implantation, and in these small numbers of patients, it does not appear that increasing time decreases the repair rate.12 The MitraClip system is designed to deliver the MitraClip to the mitral leaflets and is comprised of a steerable and deflectable guide, a Clip Delivery System, and a control handle (Figure 11–5). The steerable guide has a diameter of 24F at the skin entrance and tapers to 22F diameter as it crosses the atrial septum. The control knob on its handle allows the distal tip of the steerable guide to be either deflected or straightened. The steerable guide is placed within a stabilizer unit, which allows the guide to be iteratively advanced or retracted, as well as rotated. After a transseptal puncture, a stiff 0.035-inch guidewire is placed into the left atrium. Over this guidewire the steerable guide is advanced with a dilator in place. The distal tip of the dilator has a spiral groove cut into it, facilitating echocardiographic visualization. After identification of the guide into the left atrium, the dilator is removed and the guide is secured using the stabilizer unit. The MitraClip and Clip Delivery System are then inserted into the steerable guide and keyed into the precut channels within the guide, ensuring proper orientation (Figure 11–6). The Clip Delivery System is advanced until the radiopaque double markers are aligned with the distal thicker radiopaque band of the steerable guide. During this advancement, care must be taken to visualize the distal tip of the MitraClip by echocardiography to make sure that it is not inadvertently advanced out the back wall of the left atrium. Figure 11–5 The mechanical MitraClip system is controlled by knobs and levers on a complex handle system that in turn actuates wires running the length of the catheter. The proximal part contains the controls to the steerable and deflectable guide, which essentially is a 24F system to deliver the MitraClip-containing catheter to the left atrium. The middle section is the Clip Delivery System, and the distal part is the control handle.
The MitraClip Device and Procedural Overview
11.1 Key Points
11.2 Background
11.4 MitraClip System
11.5 EVEREST I and II Trials
11.6 EVEREST High-Risk Registry
Technical and Equipment Issues
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The MitraClip Device and Procedural Overview
