The Kidney in Hypertension
Renal parenchymal involvement in hypertension
In contrast to the remarkable reduction in deaths from stroke and coronary heart disease (CHD) that has been achieved primarily as a result of improved control of hypertension (6.1), the prevalence of end-stage renal disease (ESRD) in patients with hypertension increases unabated (6.2). Some have ascribed this phenomenon to the remarkably increased prevalence of diabetes mellitus and obesity. In part, it reflects broader defined limits to abnormal glucose levels and carbohydrate tolerance as well as for overweight/obesity. But we must also take cognizance of the fact that the incidence of hypertensive disease in patients with diabetes mellitus is extremely high.
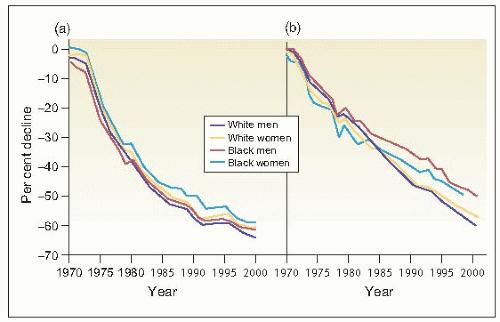 6.1 Reduction in age-adjusted mortality rates for (a) stroke and (b) coronary heart disease by gender and race in the United States. |
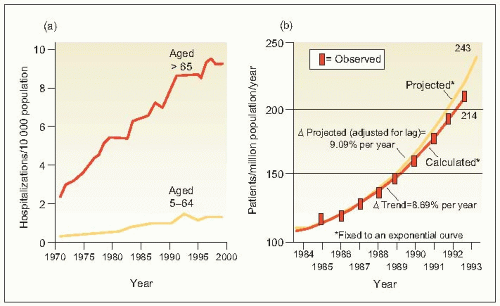 6.2 (a) Hospitalization rates for congestive heart failure and (b) observed and projected treated incidence rates for end-stage renal disease (ESRD) in the United States. |
Hypertension is extremely common in the general population of acculturated societies (prevalence over 20%), but is much higher in blacks (about 40%) and in the elderly
(50% or more). Consequently, hypertensive disease is extremely common in patients with both diabetes and hypertension and is probably even more common in black and elderly patients with diabetes. Even in the early 1920s, when the association of hypertension and diabetes was first reported, hypertensive disease was clearly demonstrated in 50-60% of patients with hypertension over the age of 50 years. This co-occurrence is still extremely common today (> 50%), even with the use of more expanded definitions (or criteria) for hypertension, diabetes mellitus and obesity (alone and/or their mutual coexistence). The point is not to denigrate the popular emphasis on these strong and close relationships, but simply to point out that antihypertensive therapy has not yet led to a reduction in the complication of ESRD as dramatic as the reduction achieved in death rates from stroke and CHD.
(50% or more). Consequently, hypertensive disease is extremely common in patients with both diabetes and hypertension and is probably even more common in black and elderly patients with diabetes. Even in the early 1920s, when the association of hypertension and diabetes was first reported, hypertensive disease was clearly demonstrated in 50-60% of patients with hypertension over the age of 50 years. This co-occurrence is still extremely common today (> 50%), even with the use of more expanded definitions (or criteria) for hypertension, diabetes mellitus and obesity (alone and/or their mutual coexistence). The point is not to denigrate the popular emphasis on these strong and close relationships, but simply to point out that antihypertensive therapy has not yet led to a reduction in the complication of ESRD as dramatic as the reduction achieved in death rates from stroke and CHD.
Thus, it may legitimately be asked why the prevalence of ESRD continues to increase and why target organ involvement of the kidney has not been reduced by antihypertensive treatment. Various explanations have been proposed (6.3).
Table 6.1 Pathophysiological mechanisms favouring development of ESRD in hypertension and diabetes | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 6.3 Reasons why, of target organ involvement in hypertension, that of the kidney is not reduced by treatment. I favour none of these; but arguments presented herein offer other ideas. |
Despite the fact that antihypertensive therapy has failed to halt the increase in prevalence of ESRD, several wellcontrolled multicentre trials have shown that reducing target blood pressure in patients with diabetes results in improved renal outcomes. Consequently, all national and international guidelines now recommend that treatment should aim to reduce arterial blood pressure to < 130 and < 80 mmHg (systolic and diastolic, respectively) in all patients with diabetes mellitus or with hypertension-associated renal parenchymal functional involvement, as soon as this becomes apparent. Despite this, there remains no adequate explanation for the continued increase in ESRD.
Several major pathophysiological alterations have been proposed to explain the underlying mechanisms of hypertensive vascular disease or of diabetes mellitus that favour the development of ESRD (Table 6.1). These alterations are extremely common in patients with hypertension and/or with diabetes mellitus as well as in elderly normotensive or even non-diabetic patients. Therefore, it is clear that ageing is one major factor that is associated with renal functional impairment not only in patients with hypertension or diabetes mellitus, but also in those who are otherwise normal.
Pathophysiological changes
The renal vasculature is extremely vulnerable to increases in arterial pressure, particularly as a consequence of systemic arterial as well as glomerular arteriolar vasoconstriction. Initially, the afferent glomerular arterioles become more prominently constricted but, as the disease progresses and as arterial pressure increases further, the efferent glomerular arterioles also become progressively constricted. Associated with this renal arterial and glomerular arteriolar constriction, the walls of the intrarenal and glomerular vessels
undergo hyaline degeneration, develop progressive sclerosis and thicken. These changes add further to the effects of luminal narrowing and glomerular injury (6.4).
undergo hyaline degeneration, develop progressive sclerosis and thicken. These changes add further to the effects of luminal narrowing and glomerular injury (6.4).
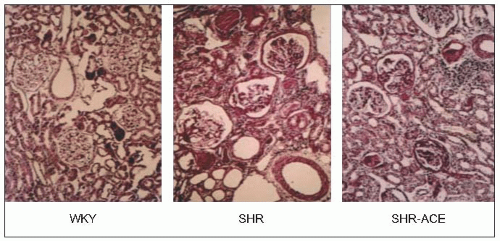
Clinical estimates of intrarenal haemodynamic alterations initially utilized the Gomez formulae, which combine measurements of renal plasma flow, glomerular filtration rate, haematocrit and plasma protein concentration. However, with the subsequent introduction of renal micropuncture techniques, studies involving experimental animals with various models of hypertension or diabetes provided more direct measurements of the renal haemodynamic and glomerular dynamic alterations. These techniques have been employed in rats with naturally developing genetic hypertension, so-called spontaneously hypertensive rats (SHRs), and in their normotensive Wistar-Kyoto controls (WKY).
 6.5 Haemodynamics and renal function in spontaneously hypertensive rats (SHRs) with naturally developing end-stage renal disease, control Wistar-Kyoto rats (WKY) and SHRs treated with an angiotensin-converting enzyme inhibitor (ACE). (a) Mean arterial pressure. (b) Estimated renal plasma flow. (c) Glomerular filtration rate. (d) Filtration fraction. (e) Renal vascular resistance. *P < 0.01 compared with Wistar-Kyoto rats. †P < 0.01, ††P < 0.05 compared with SHRs. |
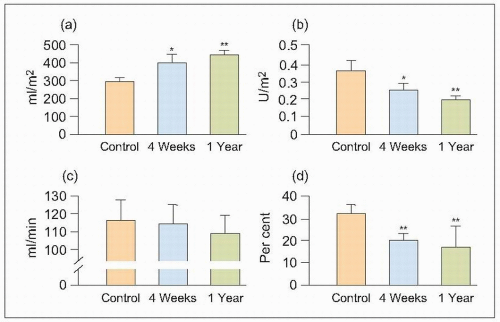
It was found that ESRD develops naturally with ageing (73 weeks) in SHRs, and the findings confirm the earlier pathophysiological changes described in other experimental models (which required more complex interventions with the kidney, the addition of chemicals and drugs, etc.) as well as more indirect measurements in humans. Thus, as mean arterial pressure (MAP) rises, and as afferent and efferent arteriolar constriction progressively increase, the estimated total renal blood flow (ERBF), glomerular filtration rate (GFR) and their ratio, the renal filtration fraction (FF), diminish (6.5).
Consequently, the single-nephron plasma flow (SNPF), single-nephron glomerular filtration rate (SNGFR) and single-nephron filtration fraction (SNFF) diminish as a result of the coincident increases in renal afferent (RA) and efferent (RE) resistance and glomerular arteriolar resistance.
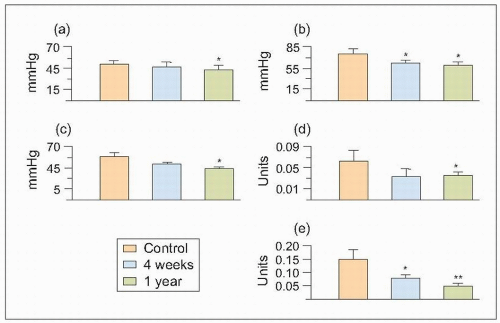
These altered glomerular dynamics result in increased intraglomerular arteriolar pressure (RG) and increased hyperfiltration and FF. These changes favour the pathological changes associated with ischaemia and ultrafiltration and result in the leakage of plasma components across the matrix production by vascular smooth muscle cells glomerular capillaries and also, probably, from the intrarenal vasculature into the renal interstitium (6.6).
These altered glomerular dynamics result in increased intraglomerular arteriolar pressure (RG) and increased hyperfiltration and FF. These changes favour the pathological changes associated with ischaemia and ultrafiltration and result in the leakage of plasma components across the matrix production by vascular smooth muscle cells glomerular capillaries and also, probably, from the intrarenal vasculature into the renal interstitium (6.6).
Renal histological studies in 73-week-old SHRs suggest that the severe nephrosclerosis that naturally develops in this model is very similar to that observed more indirectly (using the Gomez formulae) in patients with essential hypertension and ESRD. This suggests that the Gomez formulae are a valid tool and, indeed, these formulae have been used to demonstrate the long-term efficacy of the calcium antagonist diltiazem in patients with arterial disease attributable to essential hypertension (6.7 and 6.8).
 6.6 Glomerular dynamics in naturally developing end-stage renal disease in aged spontaneously hypertensive rats (SHRs), control Wistar-Kyoto rats (WKY) and SHRs treated with an angiotensin-converting enzyme inhibitor (ACE) obtained by renal micropuncture. (a) Single-nephron plasma flow. (b) Single-nephron glomerular filtration rate. (c) Single-nephron filtration fraction. (d) Intraglomerular arterial pressure. (e) Afferent resistance. (f) Efferent resistance. *P < 0.01, **P < 0.05 compared with Wistar-Kyoto rats. †P < 0.01, ††P < 0.05 compared with SHRs. |
Table 6.2 Glomerular and arteriolar injury scoring | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
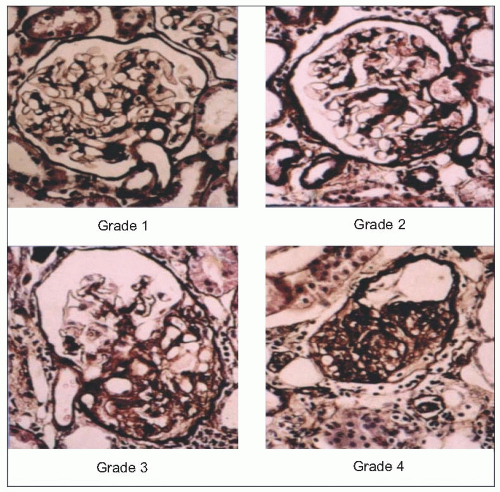
The results of micropuncture measurements are very closely correlated with the microscopic findings of severe arteriolar and glomerular injury in SHRs and with similarly obtained data from normotensive WKY rats utilizing the arteriolar and glomerular injury scoring criteria of L. Raij and his associates (Table 6.2 and 6.9).
Remarkably, and most impressively, when these 73-week-old SHRs were treated for only 3 weeks with an angiotensin-converting enzyme (ACE) inhibitor, the abnormal patho-physiological measurements obtained by micropuncture were dramatically reversed (6.10). Furthermore, these pathological changes were accompanied by a significant reduction in proteinuria (6.11). Thus, although the physiological measurements obtained by micropuncture were obtained from the superficial glomeruli with minimal pathological changes (i.e. from less diseased nephrons), the significant (parallel) changes confirm the findings from histological studies of sections of the juxtamedullary glomeruli that cannot be reached by micropuncture in the deeper tissues of the kidney (6.12).
 6.10 Glomerular and arteriolar scoring in spontaneously hypertensive rats (SHRs), control Wistar-Kyoto rats (WKY) and SHRs treated with the angiotensin-converting enzyme inhibitor (ACE) quinapril using the Raij criteria. (a) Arteriolar injury score. (b) Glomerular injury score. (c) Nephrosclerosis score. *P < 0.0001 compared with Wistar-Kyoto rats. †P < 0.0001 compared with spontaneously hypertensive rats (ANOVA). |
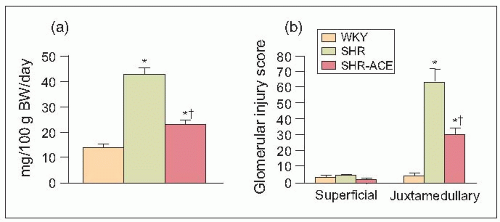 6.11 Relationships between (a) 24-hour urinary protein excretion (UPE) and (b) glomerular injury score (as shown by superficial and juxtaglomerular injury scores) in 73-week-old Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHRs) with naturally developing end-stage renal disease. The SHR were either untreated or were treated with an angiotensin-converting enzyme (ACE) inhibitor for 3 weeks. |
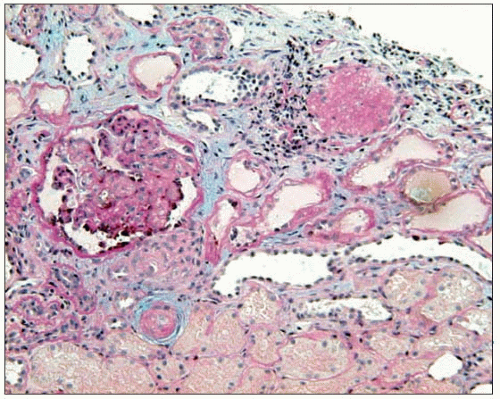
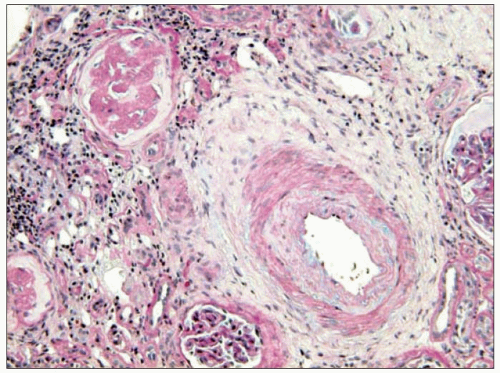
Thus, fibrinoid necrosis of the arterioles results as a consequence of the haemodynamic and glomerular dynamic alterations described above and, when the development of
the disease is exceedingly rapid and severe, the arteriolar lesions present an onion skin appearance that is pathognomonic of malignant nephrosclerosis (6.13). As non-malignant hypertensive renal involvement advances, arteriolosclerosis is exacerbated, and the glomeruli exhibit ischaemic wrinkling of the glomerular tuft, thickening of Bowman’s capsule, periglomerular fibrosis and glomerular sclerosis; tubular atrophy and interstitial fibrosis also occur.
the disease is exceedingly rapid and severe, the arteriolar lesions present an onion skin appearance that is pathognomonic of malignant nephrosclerosis (6.13). As non-malignant hypertensive renal involvement advances, arteriolosclerosis is exacerbated, and the glomeruli exhibit ischaemic wrinkling of the glomerular tuft, thickening of Bowman’s capsule, periglomerular fibrosis and glomerular sclerosis; tubular atrophy and interstitial fibrosis also occur.
These pathophysiological changes have been studied in our laboratory under tightly controlled conditions before and after a 3-week course of antihypertensive therapy using the meticulous single-nephron micropuncture techniques described above in various experimental models of hypertension. We found that administration of an ACE inhibitor significantly reduced the arterial and glomerular injury scores (the sum of these two injury indices represents a nephrosclerosis score) (6.10) accompanied by a remarkable decrease in urinary protein excretion (6.11) and, of course, by physiological renal haemodynamic and glomerular dynamic changes (6.5 and 6.6).
One major disadvantage of the above experimental studies is the need to allow SHRs to age for almost 2 years, a very costly and time-consuming practice. As one factor that is common to SHRs and clinical hypertension, ageing, atherosclerosis and nephrosclerosis (6.14) is the development of endothelial dysfunction, we repeated the above studies in much younger, 17-week-old, SHRs, but this time adding the nitric oxide synthetase enzyme inhibitor L-NAME (nitro-L-arginine methyl ester hydrochloride) to the drinking water for 3 weeks. Thus, SHRs received tap water only (control), or tap water containing L-NAME with or without the same ACE inhibitor given to the 73-week-old SHRs (6.15). An extremely interesting finding was that 17-week-old SHRs administered L-NAME for only 3 weeks exhibited all of the pathophysiological changes of ESRD that occurred naturally with ageing in 73-week old SHRs (6.14, 6.15, 6.16, 6.17, 6.18, 6.19, 6.20).
 6.14 Endothelial dysfunction of the renal arteries, arterioles and glomerular vessels is common to the nephrosclerosis of hypertension, diabetes, ageing and atherosclerosis. |
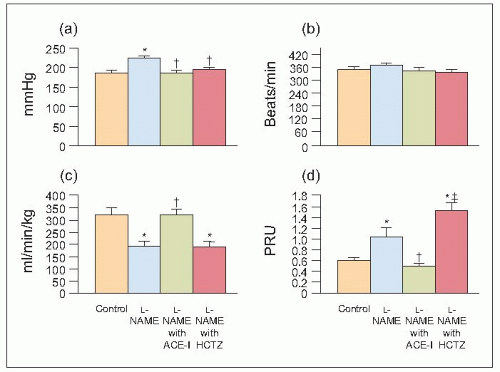 6.15 Systemic haemodynamic changes in control and L-NAME-treated spontaneously hypertensive rats (SHRs) and L-NAME-treated SHRs also administered an ACE inhibitor or hydrochlorothiazide (HCTZ). (a) Mean arterial pressure. (b) Heart rate. (c) Cardiac index. (d) TPRI. *P < 0.05 compared with control. †P < 0.05 compared with L-NAME. ‡P ≤ 0.01 compared with angiotensin-converting enzyme inhibitor (ACE-I). |
Thus, when the same ACE inhibitor was co-administered with L-NAME to younger (20-week-old) adult SHRs, the pathophysiological alterations were identical to those observed in the earlier studies of aged (73-week-old) SHRs (6.15,6.16,6.17,6.18,6.19,6.20). The systemic haemodynamic (6.15), glomerular dynamics and intrarenal haemodynamics were reversed (6.16) pathologically (6.17), clinically, (6.18), histologically (6.19) and immunologically (6.20). Of particular note, when another series of younger adult SHRs were treated with a diuretic, hydrochlorothiazide, the dramatic improvements observed in the ACE inhibitortreated group were not achieved. In fact, further deterioration of renal parenchymal, haemodynamic and dynamic function (6.15,6.16,6.17,6.18,6.19,6.20) that was the complete opposite of the changes observed in animals treated with ACE inhibitor and the diuretic was confirmed pathologically (6.17). Furthermore, in a very recent study, the adverse pathophysiological changes produced by the diuretic were totally prevented by the addition of an ACE inhibitor or an angiotensin II (type 1) receptor blocking agent or both.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree