The Fontan Principle
The Fontan principle is applied to patients with single-ventricle congenital heart disease. The ultimate goal is to achieve a circulation in which the systemic venous return is delivered directly to the pulmonary arteries, and the single ventricle is used for systemic blood flow. The original Fontan operation utilized an atriopulmonary connection for patients with tricuspid atresia. Since then, the procedure and its indications have evolved, allowing for a Fontan circulation in many single-ventricle patients.
Pathophysiology of a Single Ventricle
The patient with a single ventricle may present in a variety of ways depending on the presence or absence of obstruction to pulmonary or systemic flow. Severe obstruction to pulmonary flow results in cyanosis. Obstruction to systemic flow may lead to inadequate systemic perfusion and a low cardiac output state. The flow of blood through a patent ductus arteriosus bypasses the obstruction in either the pulmonary or systemic circulation, maintaining a clinically stable state. However, when the ductus starts to close, clinical deterioration becomes evident. In a small group of patients, there is no or minimal obstruction to systemic or pulmonary blood flow. Initially, these patients may demonstrate well-balanced pulmonary and systemic circulations. However, as the pulmonary vascular resistance diminishes over the first several weeks of life, pulmonary blood flow increases and congestive heart failure develops. If pulmonary venous obstruction is present, the patient may be cyanotic owing to increased pulmonary vascular resistance.
Management of the neonate with a single ventricle is directed at achieving adequate systemic oxygenation while preventing the development of pulmonary vascular disease. Surgical intervention may be required to achieve unimpeded outflow from the single ventricle into the systemic circulation. Adequate mixing of systemic and pulmonary venous blood must be ensured. These hemodynamic parameters allow the patient to become a candidate for a subsequent Fontan procedure.
Surgical Management
Infants younger than 3 months with inadequate pulmonary blood flow require a systemic-pulmonary artery shunt (see Chapter 18). Infants with excessive pulmonary blood flow with no obstruction to systemic outflow require early intervention aimed at reducing the volume load on the systemic ventricle and reducing pulmonary blood flow to prevent pulmonary vascular disease. In the past, pulmonary artery banding has been used to accomplish these goals (see Chapter 16). However, pulmonary artery banding may not limit pulmonary blood flow sufficiently or may result in distortion of the right or both pulmonary arteries. Therefore, many surgeons believe that division and oversewing of the proximal main pulmonary artery with construction of a systemic-pulmonary shunt is the best palliation in these cases. Patients who have both excessive pulmonary blood flow and obstruction to systemic outflow are best treated with a combined Damus-Kay-Stansel procedure and a shunt (see Chapter 30).
Management after the Neonatal Period
The goal in these patients is to minimize both the pressure and volume load on the single ventricle as soon as possible. All patients should undergo routine cardiac catheterization at 4 to 6 months of age. If signs or symptoms of ventricular dysfunction, atrioventricular valve problems, or increased pulmonary vascular resistance are noted, the study should be performed earlier. These patients are prone to develop aortopulmonary collateral vessels. Therefore, during cardiac catheterization, a search for collateral vessels should be made, and, if present, they should be occluded with coils.
Any aortic arch or subaortic obstruction that has not been dealt with previously must be corrected before proceeding with any other surgical interventions. Subaortic obstruction may require a Damus-Kay-Stansel procedure (see Chapter 30) or enlargement of the bulboventricular foramen. Aortic arch obstruction or discrete coarctation may respond to balloon angioplasty or may require surgical intervention (see Chapters 15 and 29). Any situation that requires the single ventricle to pump blood to
both the systemic and pulmonary circulations puts a so-called volume load on that ventricle. All single-ventricle complexes initially have an extra volume load on the ventricle. This is true whether the pulmonary blood flow is provided through a systemic-pulmonary artery shunt, or through controlled forward flow from the single ventricle, as is seen with pulmonic stenosis or following a pulmonary artery banding procedure. A superior cavopulmonary connection removes the volume load from the ventricle because all pulmonary blood flow is directly from the superior vena cava. The ventricle provides forward flow into the systemic circulation only. This procedure can be performed successfully once the elevated pulmonary vascular resistance has fallen, usually by 3 months of age. Early removal of the volume load on the ventricle will potentially improve its long-term function.
both the systemic and pulmonary circulations puts a so-called volume load on that ventricle. All single-ventricle complexes initially have an extra volume load on the ventricle. This is true whether the pulmonary blood flow is provided through a systemic-pulmonary artery shunt, or through controlled forward flow from the single ventricle, as is seen with pulmonic stenosis or following a pulmonary artery banding procedure. A superior cavopulmonary connection removes the volume load from the ventricle because all pulmonary blood flow is directly from the superior vena cava. The ventricle provides forward flow into the systemic circulation only. This procedure can be performed successfully once the elevated pulmonary vascular resistance has fallen, usually by 3 months of age. Early removal of the volume load on the ventricle will potentially improve its long-term function.
By performing the Fontan connection in two stages, the operative risk for the completion Fontan operation has been reduced. When the volume load is acutely removed from the ventricle, the afterload of the single ventricle has been shown to increase. This afterload increasing effect is smaller when only the superior vena cava is connected to the pulmonary artery as compared to the Fontan procedure in which all systemic venous return is diverted into the pulmonary artery. The staged approach lessens the impact of afterload mismatch at each stage. The decrease in diastolic ventricular volume is also less with the superior cavopulmonary connection. By staging the Fontan, the sometimes fatal combination of ventricular hypertrophy and a sudden decrease in diastolic volume can be avoided.
Bidirectional Glenn Procedure
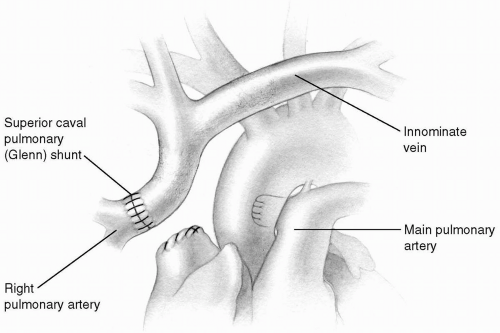
The classic cavopulmonary, or Glenn, shunt achieved by anastomosing the transected superior vena cava to the transected right pulmonary artery is rarely performed today (Fig. 31-1). The bidirectional superior cavopulmonary anastomosis, or bidirectional Glenn, allows superior vena caval return to enter both the right and left pulmonary arteries. Because only 40% to 50% of the systemic venous return is presented to the pulmonary arterial bed, patients who would not be candidates for a full Fontan procedure may be able to undergo a bidirectional Glenn shunt. This procedure is often used as part of a staged approach for the patient with a single ventricle. The bidirectional Glenn may also be used in patients with small or dysfunctional right ventricles to create a so-called one and one-half ventricle repair. This may allow some patients who are not candidates for a two-ventricle repair to have the right ventricle manage part of the systemic venous return.
Cannulation
A standard median sternotomy is performed. A bidirectional Glenn may be performed without cardiopulmonary bypass, using a shunt between the most proximal aspect of the superior vena cava and the right atrial appendage. In this case, two right-angled venous cannulas are selected, approximating the size of the superior vena cava. Purse-string sutures are placed at the superior vena cava-innominate vein junction and in the right atrial appendage. Systemic heparin is then administered, after which the superior vena caval cannula is placed. The cannula is allowed to fill with blood and clamped. A second cannula is then placed in the right atrial appendage. The blood from the right atrium is allowed to fill this cannula, which is then connected to the first cannula, making sure no air is trapped in the connector. The shunt is opened to allow flow from the superior vena cava into the right atrium. Any previously placed systemic to pulmonary artery shunts are dissected circumferentially. The azygos vein is doubly ligated with fine silk ties and
divided between the ligatures to allow full mobilization of the superior vena cava. A tape around the superior vena caval cannula is now snared, and an angled vascular clamp is placed just above the right atrium-superior vena cava junction. The superior vena cava is then transected (Fig. 31-2). The right atrium-superior vena cava junction is oversewn with a running 6-0 Prolene suture, and the vascular clamp is removed.
divided between the ligatures to allow full mobilization of the superior vena cava. A tape around the superior vena caval cannula is now snared, and an angled vascular clamp is placed just above the right atrium-superior vena cava junction. The superior vena cava is then transected (Fig. 31-2). The right atrium-superior vena cava junction is oversewn with a running 6-0 Prolene suture, and the vascular clamp is removed.
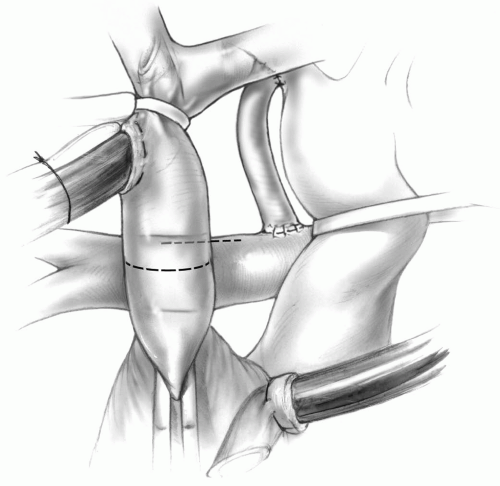 FIG 31-2. Bidirectional Glenn shunt: Transection of the superior vena cava and a longitudinal opening on the superior aspect of the right pulmonary artery. |
To prevent twisting of the proximal superior vena cava after transection, the azygos vein may be simply ligated or occluded with a metal clip. This maintains the correct orientation of the superior vena cava during its anastomosis to the pulmonary artery. Alternatively, marking sutures may be placed on the superior vena cava to maintain the orientation of the vessel during the anastomosis.
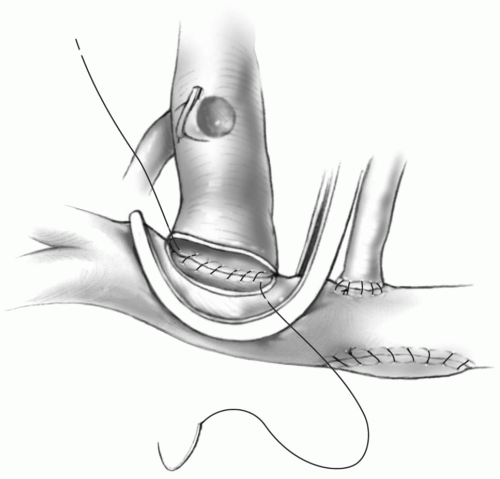
The superior aspect of the right pulmonary artery is grasped with a curved clamp, and an opening on the superior aspect of the right pulmonary artery is made with a knife blade and Potts scissors. The anastomosis of the superior vena cava to the right pulmonary artery is then accomplished with a running 6-0 or 7-0 Prolene suture beginning at the most medial aspect of the pulmonary arteriotomy, completing the posterior row with one needle, and then the anterior aspect with the second needle (Fig. 31-3).
 If the bidirectional Glenn is to be performed off pump, the source of pulmonary blood flow must be maintained during the construction of the anastomosis. If the pulmonary flow is from the ventricle through a native valve, pulmonary band, or ventricular-pulmonary shunt, placement of the clamp on the right pulmonary artery should be well tolerated. However, if a systemic-pulmonary shunt to the right pulmonary artery is present, the clamp on the right pulmonary artery must be placed carefully. Unless the previous shunt is centrally located on the right pulmonary artery, it may not be possible to perform the bidirectional Glenn without cardiopulmonary bypass.
If the bidirectional Glenn is to be performed off pump, the source of pulmonary blood flow must be maintained during the construction of the anastomosis. If the pulmonary flow is from the ventricle through a native valve, pulmonary band, or ventricular-pulmonary shunt, placement of the clamp on the right pulmonary artery should be well tolerated. However, if a systemic-pulmonary shunt to the right pulmonary artery is present, the clamp on the right pulmonary artery must be placed carefully. Unless the previous shunt is centrally located on the right pulmonary artery, it may not be possible to perform the bidirectional Glenn without cardiopulmonary bypass.Tension on the anastomosis between the superior vena cava and right pulmonary artery must be avoided by leaving the superior vena cava as long as possible and placing the opening on the right pulmonary artery as close to the transected superior vena cava as feasible. This avoids any tension on the anastomosis that may lead to intraoperative bleeding from the suture line, dehiscence of the suture line, or long-term fibrosis and narrowing of the anastomosis.
It may be prudent to use interrupted sutures on the anterior aspect of the anastomosis to prevent a purse-string effect and narrowing of the anastomosis. This is especially important if the superior vena cava is small in diameter, as is seen when bilateral superior vena cavae are present.
Completing the Shunt
The clamp on the pulmonary artery is removed, and the anastomosis is inspected for bleeding and patency.
The shunt tubing is clamped, the superior vena caval cannula is taken out, and the purse-string suture is secured. Any previously placed systemic-pulmonary or ventricular-pulmonary shunt is occluded with metal clips. If forward flow from the ventricle is present, the pulmonary artery may be tightly banded or transected and oversewn. The right atrial cannula is removed, and protamine is administered.
The shunt tubing is clamped, the superior vena caval cannula is taken out, and the purse-string suture is secured. Any previously placed systemic-pulmonary or ventricular-pulmonary shunt is occluded with metal clips. If forward flow from the ventricle is present, the pulmonary artery may be tightly banded or transected and oversewn. The right atrial cannula is removed, and protamine is administered.
The sinoatrial node is located on the lateral aspect of the junction between the atrium and superior vena cava and is prone to injury. The surgeon should place the clamp well away from this area, and suturing should be carried out with this potential complication in mind.
Ligation of the pulmonary artery creates a space between the pulmonic valve and the ligature where stasis occurs and thrombus frequently develops. The main pulmonary artery should be transected just above the valve, the pulmonic valve oversewn, and both ends closed with a running 5-0 or 6-0 Prolene suture. This requires cardiopulmonary bypass, so the presence of antegrade flow through the native pulmonary artery is a contraindication to an off pump bidirectional Glenn.
Some surgeons believe that an additional source of pulmonary blood flow is important is these patients. This can be achieved by leaving a systemic-pulmonary or ventricular-pulmonary shunt in place, with or without narrowing the conduit. If forward flow from the ventricle is present, the pulmonary artery can be tightly banded. The beneficial effects of these procedures are an increase in the oxygen saturation levels and potentially better pulmonary artery growth. The downside is increasing the volume load on the ventricle. If additional pulmonary flow is maintained, the pulmonary artery pressure must be monitored.
The incidence of pulmonary arteriovenous malformations increases with time following a bidirectional Glenn procedure. This is felt to be due to the exclusion of hepatic venous blood from the pulmonary arterial circulation. This can lead to progressive cyanosis if patients are left with the bidirectional Glenn circulation for a prolonged period of time. Because the bidirectional Glenn is most often performed as part of a staged Fontan procedure, pulmonary arteriovenous malformations are usually not an issue. Bidirectional Glenn should not be the final procedure for patients who are not candidates for a full Fontan procedure because of the significant risk of developing these pulmonary arteriovenous malformations. The restoration of hepatic venous flow to the pulmonary arterial bed leads to the regression of these malformations. Prevention of these pulmonary arteriovenous malformations is one argument made by surgeons who prefer to maintain an additional source of pulmonary blood flow when performing the bidirectional Glenn procedure.
A pulmonary artery pressure greater than 20 mm Hg will not be tolerated and this circumstance may necessitate interrupting additional sources of flow into the pulmonary artery.
If superior vena caval pressures remain high, direct needle measurements of the pressure in the pulmonary artery and superior vena cava should be made to rule out an anastomotic problem. If pulmonary artery pressures remain at 20 or above despite maneuvers to reduce pulmonary vascular resistance, the bidirectional Glenn must be taken down, the superior vena cava reanastomosed to the right atrium, and a systemic to pulmonary artery shunt performed.
If the previously placed systemic-pulmonary or ventricular-pulmonary shunt is simply occluded with a metal clip and not divided, the pulmonary artery may become distorted. It is preferable to clip the shunt tubing proximally and distally and divide it.
Simply tying down the purse-string suture at the superior vena caval cannulation site may result in significant distortion and obstruction to flow into the distal superior vena cava and pulmonary artery. If this occurs, the superior vena cava should be grasped with a shallow curved clamp, the purse-string suture removed, and the opening meticulously repaired with a running or interrupted 7-0 Prolene sutures.
Bidirectional Glenn on Cardiopulmonary Bypass
In some patients, it is necessary or preferable to perform the bidirectional Glenn shunt on cardiopulmonary bypass. This is particularly true when patients require reconstruction of the pulmonary arteries, or in patients with bilateral superior venae cavae who require bilateral bidirectional
Glenn shunts. In these cases, cannulation of the ascending aorta, very proximal superior vena cava, and right atrium is performed. Cardiopulmonary bypass is commenced, and previously placed systemic-pulmonary or ventricular-pulmonary shunts are closed. The previously described procedure for anastomosis of the superior vena cava to the right pulmonary artery can then be performed with the heart decompressed. It is rarely necessary to cross-clamp the ascending aorta and arrest the heart.
Glenn shunts. In these cases, cannulation of the ascending aorta, very proximal superior vena cava, and right atrium is performed. Cardiopulmonary bypass is commenced, and previously placed systemic-pulmonary or ventricular-pulmonary shunts are closed. The previously described procedure for anastomosis of the superior vena cava to the right pulmonary artery can then be performed with the heart decompressed. It is rarely necessary to cross-clamp the ascending aorta and arrest the heart.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree