Chapter 16 The Epidemiology of Peripheral Artery Disease
Peripheral artery disease (PAD) is generally defined as partial or complete obstruction of one or more peripheral arteries due to atherosclerosis. Although the term PAD is sometimes inclusive of all peripheral arteries, in this chapter PAD refers to atherosclerotic occlusive disease of the lower extremities. Peripheral artery disease is associated with many of the same risk factors as atherosclerotic cardiovascular and cerebrovascular diseases, and is very common among the elderly. Peripheral artery disease that exhibits typical symptomatology, usually in the form of leg pain brought about by walking, has been conservatively estimated to reduce quality of life in at least 2 million Americans, and in some cases leads to a need for surgical revascularization or amputation.1 Six million more Americans have measurable asymptomatic disease or disease with atypical symptoms.2 Both symptomatic and asymptomatic PAD have been shown to be associated with a sharply elevated risk of mortality due to coronary and cerebrovascular disease.3
Symptoms and Measures of Peripheral Artery Disease in Epidemiology
Early studies of PAD focused primarily on claudication as the chief symptomatic manifestation of PAD. A number of patient questionnaires have been developed to uniformly identify claudication and distinguish it from other types of leg pain. The first of these was the Rose questionnaire, also referred to as the World Health Organization questionnaire.4 The San Diego Claudication Questionnaire (SDCQ) is a modification of the Rose questionnaire that additionally captures information on the laterality of symptoms.5 Recently we completed an evidence-based shortened revision of the SDCQ that is shown in Table 16-1.
Table 16-1 San Diego Claudication Questionnaire (Brief Version)
| Circle Answer | |
| 1. Do you get pain or discomfort in either leg on walking? (If no, stop.) | Right leg Yes No Left leg Yes No |
| 2. Does this pain ever begin when you are standing still or sitting? | Right leg Yes No Left leg Yes No |
| 3. Does this pain include your calf/calves? | Right leg Yes No Left leg Yes No |
| 4. Do you get it when you walk at an ordinary pace on the level? | Right leg Yes No Left leg Yes No |
| 5. What do you do if you get it when you are walking? | Right leg Stop or slow down Continue on Left leg Stop or slow down Continue on |
| 6. What happens to it if you stand still? | Right leg Lessened or relieved Unchanged Left leg Lessened or relieved Unchanged |
Determine pain category separately for each leg as follows:
1. No pain: 1 = no
2. Pain at rest: 1 = yes and 2 = yes
3. Non-calf: 1 = yes and 2 = no and 3 = no
4. Classic: 1 = yes and 2 = no and 3 = yes and 4 = yes and 5 = stop or slow down and 6 = lessened or relieved
5. Atypical calf: 1 = yes and 2 = no and 3 = yes and not classic
Ankle-Brachial Index
Although intermittent claudication is an important manifestation of PAD, it is not pathognomonic. Atherosclerosis may have been developing for many years before claudication begins, and the extent to which it occurs is influenced by factors other than disease per se, such as the patient’s level of activity.6 Furthermore, the definitional distinctions used to separate claudication from other leg pain make claudication more specific to arterial disease but less sensitive to other types of pain that may in some cases be related to arterial disease. Spinal stenosis can cause leg pain similar to claudication during exercise.
For these reasons, another method of diagnosing PAD was needed. Low blood pressure at the ankle was proposed as a test for PAD as early as 19507 and led to development of a simple measure called the ankle-brachial index (ABI). The ABI is the ratio of the systolic blood pressure at the ankle to that in the arm. An abnormally low ABI is indicative of atherosclerosis of the lower extremities. The ABI has been shown to have good receiver operating curve characteristics as a test for PAD. Although there is no definitive cut point above which disease is always absent and below which disease is always present, an ABI of 0.9 or less is commonly used in both clinical practice and epidemiological research to diagnose PAD. The ABI is also sometimes referred to as the ankle-brachial pressure index (ABPI)8 and the ankle-arm index (AAI).9
As a test for ABI-based PAD, claudication has been shown to have very high specificity but very low sensitivity. For example, in the Rotterdam Study, 99.4% of subjects with ABI 0.9 or greater did not have claudication, but only 6.3% of subjects with ABI of less than 0.9 had claudication.10 In a study of elderly women in the United States, the percentages were 93.3% and 18.3%, respectively.6 Peripheral artery disease based on ABI criteria is much more common than claudication in the general population, and large numbers of patients without claudication can be shown to have either atypical or no symptoms in the presence of PAD based on ABI.
To validate the ABI and the huge burden of previously unrecognized asymptomatic disease it implied, early studies compared the ABI-based diagnosis with angiography, which was considered the gold standard for visualizing atherosclerosis in the legs. Two such studies often cited reported the sensitivity and specificity of the ABI in the 97% to 100% range.11,12 However, because angiography presents some risk to patients, it was not ethical to perform angiography on patients not suspected to have PAD, so these studies involved comparisons of patients with angiographically confirmed PAD with young healthy patients assumed not to have PAD. The sensitivities and specificities calculated are therefore based on the ability of the ABI to discriminate between extremes of disease and wellness. If measured among patients seen in routine clinical practice or the population in general, the specificity of the ABI remains in the 97% + range, but the sensitivity is somewhat less—closer to 80%13—in part due to some PAD patients with stiff peripheral arteries and false-negative ABIs.14
The ABI has been demonstrated to have strong associations with cardiovascular disease risk factors and disease outcomes. In the Cardiovascular Health Study (CHS) cohort, a dose-response relationship was demonstrated between ABI and cardiovascular disease risk factors, as well as both clinical and subclinical cardiovascular disease.15 In a study in Edinburgh, asymptomatic patients with an ABI of less than 0.9 were shown to have a higher risk of developing claudication and higher mortality.16 In a clinical study, patients with an ABI of less than 0.9 who did not have exertional leg pain were shown to have poorer lower-extremity functioning even after adjustment for traditional risk factors and comorbidities.17 The ABI correlates with ability to exercise as measured on an accelerometer,18 and an ABI of less than 0.6 is related to development of walking impairment.19 Thus, even aside from its association with claudication, the ABI is related to the types of functional outcomes, risk factors, and associated diseases that one would expect of a measure of PAD. The ABI has also been shown to have high intra- and inter-rater reliability.20
In practice, the ABI is measured using a blood pressure cuff, a standard sphygmomanometer, and a Doppler instrument to detect pulses. Pressure measurements are made with the patient at rest in a supine position for 5 minutes prior to measurement. Ankle pressure is measured in both legs at the dorsalis pedis and posterior tibial arteries. The higher pressure measurement in each ankle has traditionally been used as the numerator of the ABI for that ankle. Using the lower or average pressure can substantially change estimates of PAD prevalence; one study reported 47% prevalence based on the higher pressure versus 59% based on the lower.20 Results of two recent studies support the use of the average of dorsalis pedis and posterior tibial pressures as the ankle pressure for each leg, based on superior reproducibility in repeated tests and closer statistical association with leg function.20,21 However, the relative predictive value of the higher versus the average (or perhaps the lower) of the two ankle pressures for clinical events has not yet been evaluated. Practice also differs as to the brachial pressure used as the denominator of the ABI; the same brachial pressure is usually used for both left and right ABIs in the same patient, but that pressure may be the right arm, the average of both arms, or the highest of both arms. A recent study supports use of the average of the left and right arms, based on superior reproducibility,21 but another study shows a strong correlation between PAD and subclavian stenosis, suggesting the highest arm pressure should be used in the ABI calculation.22 Another issue is that the first arm pressure measured is typically higher because of the “white coat” effect, and a repeat of the first arm pressure after the other pressures are complete will often give a more accurate reading. Based on the numerators and denominators described, separate ABIs are calculated for the left and right legs of each subject. In epidemiological analyses, the unit of analysis is either the leg, with appropriate statistical adjustments for intrasubject correlation, or the subject, with disease status classified based on the “worst” limb (i.e., the limb with the lowest ABI).
The ABI has several limitations as a measure of PAD. Occlusive disease distal to the ankle is not detected by the ABI; other measures, such as pressure ratios using pressures measured in the toe, are required for detecting such distal disease. The ABI is also sensitive to the height of the patient, with taller patients having slightly higher ABIs; it is unlikely these differences are related to real differences in PAD.23,24 Similarly, it has been noted in several studies that the ABI of the left leg tends to be slightly lower on average than the ABI of the right leg.23,24 Recent data also document that ABIs in normal subjects, on average, are slightly lower in women and African Americans.25
Arterial calcification (medial calcinosis) can make the arteries of the ankle incompressible and lead to artificially high ABI values. This is particularly common in patients with diabetes.26,27 Ankle-brachial index values above 1.5 are often excluded in epidemiological analyses and should be viewed with suspicion clinically.6,15,28–30 In two large population-based studies in the United States, the proportion of patients with such elevated values was around 0.5%.15,30 Some investigators use the more conservative cut point of 1.3. New evidence suggests 1.4 may be a good compromise.31,32
Incidence and Prevalence of Peripheral Artery Disease
Although uncommon among younger people, the prevalence of PAD rises sharply with age to include a substantial proportion of the elderly population. Figure 16-1 shows some ABI-based estimates of PAD prevalence by age from six large studies.10,15,33–36 In four of the studies, the standard ABI of less than 0.9 criterion was used; in the Limburg Study, PAD was diagnosed based on two ABI measurements of less than 0.95,35 whereas in the Rancho Bernardo Study, a combination of a conservative ABI cut point of 0.8 and other noninvasive tests was used.33 Although estimates vary, prevalence appears to be well under 5% before age 50, around 10% by age 65 and in excess of 25% in patients 80 years of age or older. All studies show this stronger-than-linear relationship of prevalence to age, although there is some variability in the age at which prevalence begins to increase most dramatically.
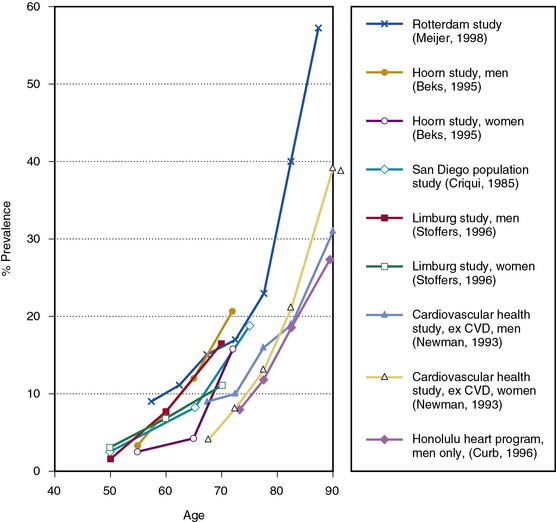
Figure 16-1 Peripheral artery disease (PAD) prevalence.
Estimates based on ankle-brachial index (ABI) from six large studies.
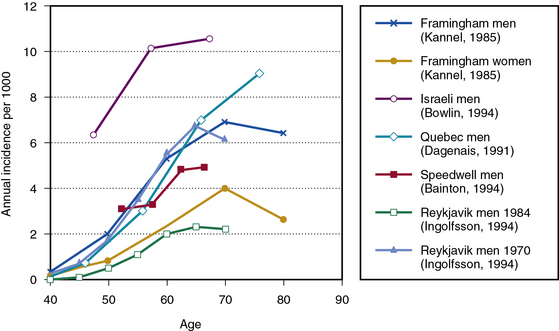
Estimates of PAD incidence are reported somewhat less frequently in the literature, with more data based on claudication incidence than on ABI. With respect to claudication, data from the Framingham Study show claudication in men rising from less than 0.4 per 1000 per year in men aged 35 to 45 years to more than 6 per 1000 per year in men aged 65 years and older.37 Incidence among women ranged from 40% to 60% lower by age, although estimates in men and women were similar by age 65 to 74. In a group of Israeli men, incidence of claudication ranged from 6.3 per 1000 per year at ages 40 to 49 to 10.5 per 1000 at age 60 and greater.1 In a study of 4570 men from Quebec, claudication incidence rose from 0.7 per 1000 per year at ages 35 to 44, to 3 per 1000 per year at ages 45 to 54, 7 per 1000 per year at ages 55 to 63, and 9 per 1000 at age 65 and greater.38 In the Speedwell Study that followed English men aged 45 to 63 years for 10 years, claudication incidence per 1000 per year ranged from 3.1 in the youngest to 4.9 in the oldest age group, based on age at baseline examination.39 A higher incidence of 15.5 per 1000 per year was reported among men and women aged 55 to 74 in the Edinburgh Artery Study; however, this study did not apply strict Rose criteria for probable claudication.40
In the Reykjavik Study, Ingolfsson et al. used Poisson regression techniques to conclude that intermittent claudication rates among Icelandic men dropped significantly between 1968 and 1986. Among 50-year-old men, their estimate of the rate of claudication dropped from 1.7 per 1000 per year in 1970 to 0.6 per 1000 per year in 1984, whereas in 70-year olds, the rate of claudication dropped from 6.0 to 2.0.41 The authors attributed this to decreased smoking and cholesterol levels. The design and duration of this study were uniquely suited to estimating long-term trends in disease incidence; comparable studies for other populations are unavailable. The potential for trends of this magnitude should be considered in reviewing results of other studies. Figure 16-2 shows incidence rates by age for various studies identifying PAD based on claudication.1,37–39,41
There are very few ABI-based studies of PAD incidence, given the time and resources required to periodically retest study subjects for incident disease. In the Limburg PAOD Study, incidence rates for PAD were based on two ABI measurements of less than 0.95. Among men, annual incidence was 1.7 per 1000 at ages 40 to 54; 1.5 per 1000 at ages 55 to 64; and 17.8 per 1000 at ages 65 and greater. Annual incidence in women was higher: 5.9, 9.1, and 22.9 per 1000, respectively, for the same age groups.42
Claudication incidence and prevalence have usually been found to be higher in men than women. For example, in the Framingham Study, annual claudication incidence for all ages combined was 7.1 per 1000 in men versus 3.6 per 1000 in women, for a male-to-female ratio of 1.97.37 In the Framingham Offspring Study, claudication prevalence was 1.9% in men versus 0.8% in women (ratio = 2.38), whereas in the Rotterdam Study it was 2.2% in men versus 1.2% in women (ratio = 1.83).10,30 However, the Edinburgh Artery Study and the Limburg PAOD Study found much lower male-to-female ratios of claudication prevalence of 1.11 and 1.2, respectively.23,35
The case for an excess of disease among males is even weaker for PAD diagnosed based on ABI. This is true even in those studies finding a clear male excess with respect to claudication. For example, in the Framingham Offspring Study mentioned earlier, PAD based on ABI was found in 3.9% of men and 3.3% of women, for a ratio of 1.18.30 In the Rotterdam Study, ABI-based PAD was actually lower in men than in women, with prevalences of 16.9% and 20.5% for a ratio of 0.8210. The Limburg PAOD Study, which reported a low male-to-female ratio for claudication, reported a similarly low ratio of 1.1 for ABI-based PAD.35 A population-based study from Southern Italy found prevalences of PAD based on ABI of less than 0.9 to be very similar in men and women, with male to female ratios by age of .89 to .99.43 In the CHS, ABI of less than 0.9 was somewhat more prevalent in men than women (13.8% vs. 11.4%; ratio = 1.21), but the association of disease with sex was not significant after adjustment for age and cardiovascular disease status.15 In the Atherosclerosis Risk in Communities (ARIC) Study, PAD prevalence based on ABI was actually lower in men than women among both African Americans (3.3% vs. 4.0%) and whites (2.3% vs. 3.3%).44
The greater male excess observed for symptomatic versus ABI-diagnosed disease may be related to severity of disease. A prevalence study in Southern California found that the excess of disease among males increased with severity of PAD.33 A report from the Multi-Ethnic Study of Atherosclerosis (MESA) showed PAD prevalence (ABI of less than 0.90) was the same in men and women (3.7%), but borderline values of ABI (0.90-0.99) were much higher in women (10.6% vs. 4.3%).45
Peripheral Artery Disease Risk Factors
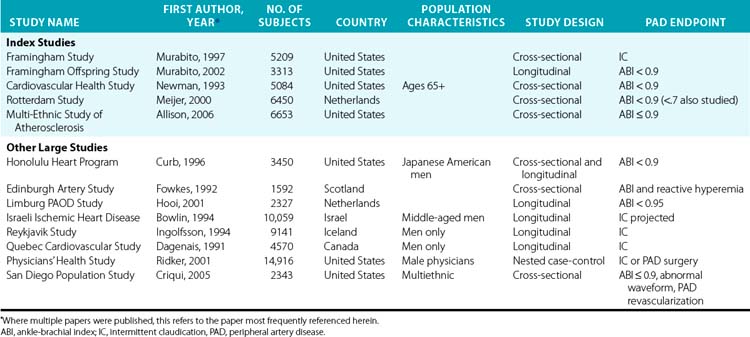
The following discussion of risk factors focuses on the results from five large epidemiological studies referred to as index studies (Table 16-2). These studies each had over 3000 subjects drawn from the general population and included both men and women. The studies are similar enough in their selection and manner of measuring risk factors and in their statistical analyses to allow reasonable comparisons for most of the common risk factors. Although the discussion draws on data from many other studies (see Table 16-2 for a partial list), data are presented from these five studies across all the conventional cardiovascular disease risk factors to provide some consistency and comparability for the reader, and as a check against potential biases that might be introduced by selecting all the studies to present for each risk factor in a more ad hoc fashion.
Smoking
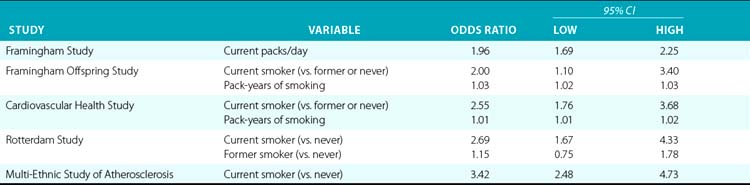
Smoking is one of the strongest risk factors for PAD in virtually all studies. Studies vary as to their measurement of smoking, often combining a categorical assessment of smoking status (current, past, or never) with some measure of current or historical volume of smoking; these multiple approaches to measurement make comparisons difficult. However, even with some type of additional adjustment for volume of smoking, current smoking versus nonsmoking has been shown to at least double the odds of PAD in most studies, with some estimates as high as a four times greater risk among smokers than others. Among the index studies, current smoking (vs. never or former/never) resulted in 2.0 to 3.4 times higher odds of PAD in the three studies using such categorization; however, in two of these studies, the models also included pack-years of smoking as a significant variable. The Rotterdam study included only current packs/day, showing a doubling of the odds of PAD for each pack a day smoked (Table 16-3). All the large population-based studies reviewed found a significant independent association between PAD and smoking.
Cessation of smoking among patients with claudication has been shown to improve various functional and physiological measures related to PAD, as well as reducing mortality.46–48 However, because symptomatic PAD patients have long been advised to quit smoking, it is possible that observational comparisons of patients who quit smoking with those who do not are confounded by other differences in compliance with medical advice between the two groups. Randomized trials of this question would raise ethical issues, but substantial bias is unlikely, given the large effect size for cigarette smoking.
Aside from the large increase in risk associated with it, smoking is the traditional risk factor for which the best case can be made for a more important role in PAD than in other atherosclerotic diseases. In a comparison of risk factors conducted in the same large cohort, Fowkes et al. found smoking to be associated with a significantly higher relative risk for PAD compared to other cardiovascular diseases. Smoking was the only traditional cardiovascular disease risk factor for which the odds ratio differed significantly between PAD and other cardiovascular diseases.49
Diabetes
Diabetes is strongly associated with an elevated risk of PAD, although the evidence for an independent role in multivariable analysis is not entirely consistent. Four of the five index studies found diabetes, dichotomized based on different criteria, to be associated with PAD after multivariable adjustment, with odds ratios ranging from 1.89 to 4.05.9,15,50,51 The Framingham Offspring Study found such an association on an age- and sex-adjusted basis, but not in multivariable models.30
Among other large population-based studies, multivariable logistic regression models have often shown a relationship to diabetes as a categorical variable,1,34,36,42,52 or various blood sugar measures as linear variables.39 Other null findings for diabetes or blood sugar measures were seen in the Edinburgh Artery Study49 and the Reykjavik Study.41
More severe and/or long-standing diabetes appears to be more strongly related to PAD. In the Hoorn Study, it was shown that known diabetes was associated with PAD in multivariable analysis, whereas newly diagnosed diabetes was only of borderline significance, and impaired glucose tolerance was not associated with PAD.34 In that study, after excluding known diabetics, none of the common glycemic indices that were tested were significantly associated with PAD based on ABI, although significant associations were observed when the PAD criteria were broadened to include patients with additional criteria. Studies conducted in patients with diabetes have shown that duration of diabetes and use of insulin are associated with PAD.53–55
Outcomes of PAD in diabetic patients have been shown to be worse. In one study, diabetic patients with PAD were five times more likely to have an amputation than other PAD patients; they also had more than three times the odds of mortality.56 There is also some evidence to support a somewhat different anatomical distribution of disease, with more disease in arteries distal to the knee in diabetic than nondiabetic persons.56,57
Lipids
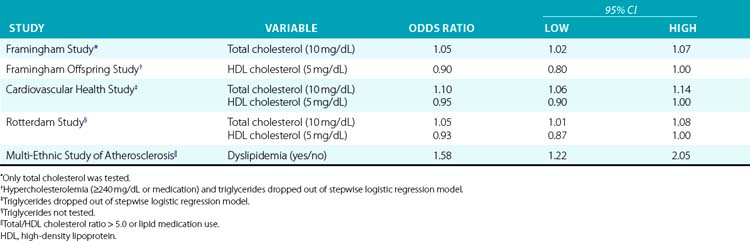
As is the case in cardiovascular disease epidemiology, the challenge of defining the roles of various lipid fractions in PAD lies in identifying the strongest independent risk factors from among multiple correlated measures. In recent studies, recognition that the ratio of total cholesterol to high-density lipoprotein (HDL) cholesterol is the best lipid measure of risk,58 along with increasing use of medication, has led to analyses that employ both these variables in the same model52 or combine the ratio with medication use in a single variable (e.g., “dyslipidemia”).51 Results from the index studies appear in Table 16-4.
Total cholesterol was the first lipid measure examined as a potential risk factor for PAD and has been the most widely studied. Total cholesterol was examined as a potential risk factor in four of the index studies, and was significantly associated with PAD in multivariable analysis in three. In the remaining study, total cholesterol was significant in univariate analysis but dropped out of multivariable models that considered other lipid measures.30 Similarly, in other studies total cholesterol has usually been found to be associated with PAD,1,36,41,49 with occasional null findings in multivariable analyses where other lipid measures are considered.39,59 One of the few null findings for total cholesterol as the sole lipid measure was an analysis of the Quebec Cardiovascular Study cohort.38
High-density lipoprotein cholesterol (HDL-C) has been shown to be protective against PAD in most studies where it was evaluated, usually in models that also considered total cholesterol. High-density lipoprotein cholesterol was included among potential risk factors in three of the five index studies and in the TC/HDL-C ratio in a fourth, and was significantly associated with PAD in multivariable analysis in all four. In two studies, both HDL-C and total cholesterol were significant in multivariable analysis, whereas in one study HDL-C but not total cholesterol was significant. Other studies have also shown a protective effect of HDL-C.36,59
Bowlin et al. found that non-HDL cholesterol (total cholesterol minus HDL cholesterol) was significantly associated with incident claudication in a large cohort of Israeli men. Neither total cholesterol nor HDL cholesterol were significantly associated with disease in models that included non-HDL cholesterol.1 In a comparison of incident cases of claudication with healthy controls in the Physician’s Health Study, Ridker et al. found that the ratio of total to HDL cholesterol was the lipid measure most strongly associated with disease, with patients in the highest quartile having 3.9 times the claudication risk of patients in the lowest quartile. Screening for other lipid fractions was judged to have little clinical usefulness beyond measurement of this ratio.60
Evidence for high triglycerides as an independent risk factor for PAD is fragmentary. Early case-control studies showed a very consistent relationship between triglycerides and PAD, suggesting a uniquely strong relationship with PAD, but large population-based cohort studies employing multivariable modeling later called this into question.49,61 Among the index studies, only two included triglycerides among the potential risk factors evaluated. In both cases, triglycerides were significant in univariate analysis but dropped out of multivariable models based on stepwise logistic regression.15,30 Similarly, in the Edinburgh Artery Study cohort and in a large study of geriatric patients in the United States, triglycerides were not significantly associated with PAD after adjustment for other lipid meaures.49,59 However, other studies have shown triglycerides to be significantly and independently associated with PAD in multivariable analysis.39,53,62 There is also some evidence suggesting that elevated triglycerides may have a special role in disease progression or more severe PAD.49,63
In summary, although total cholesterol, HDL-C, and triglycerides all appear to be associated with PAD on a univariate basis, in multivariable analysis triglycerides frequently drop out as an independent risk factor. Although it has been the most extensively studied, it is not clear that total cholesterol is the strongest independent risk factor for PAD; in one comparison of PAD patients with healthy controls, it was found that mean total cholesterol did not differ significantly, whereas triglycerides, very low-density lipoprotein (VLDL) cholesterol, low-density lipoprotein (LDL) cholesterol, HDL cholesterol, and the total-to-HDL cholesterol ratio all did.64
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree