The Diagnostic Approach to the Vascular Patient
Kim Cantwell-Gab
R. Eugene Zierler
Dr. D. Eugene Strandness Jr, fondly referred to as “DES” (according to his initials and pronounced “dezz”) by many who knew him, loved to teach about vascular disease. He believed in the traditional diagnostic process of beginning with a detailed history and complete physical examination and then choosing the physiologic or imaging tests that are best suited to answer the key clinical questions. There were many times when he would emerge from an examination room looking for surgery residents or medical students so he could show them something unique or interesting about the patient he was seeing. However, he did not limit his teaching to the residents and medical students; the vascular technologists and nurses were also important members of his team, and even the patient was often an active participant.
Patients loved Dr. Strandness and the personal interactions they had with him. We were fortunate to work alongside Dr. Strandness over many years in the vascular surgery clinic, the operating room, and on research projects, and he had a knack for remembering every patient’s name and something personal about them. He would say, “Listen to your patients. At least 90% of your diagnosis is based on the history and physical examination. The other 10% depends on diagnostic tests.” In this chapter, we discuss Dr. Strandness’ approach to obtaining a patient’s history and how he performed a vascular physical examination. In addition, we will include a few of his patient stories, because those are what many students, residents, fellows, and technologists fondly remember about their training with Dr. Strandness. The various diagnostic studies performed in each vascular testing area are covered in subsequent chapters.
Dr. Strandness was always quick to point out that patients traditionally enter the health care system only when their symptoms become significant enough for them to seek medical advice. Before performing any vascular laboratory tests, it is important to review the patient’s presenting symptoms and risk factors and include that information with the request for diagnostic studies. This is important because it provides a specific indication for the test being done and assists the vascular technologist in determining the most appropriate diagnostic test to answer the clinical questions and establish a diagnosis.
PERIPHERAL ARTERIAL DISEASE
Atherosclerosis is a systemic disease that involves multiple arterial territories in the body, so the risk factors are generally the same regardless of where and how it presents. Atherosclerosis is also the most common cause of arterial disease of the lower extremities, and a variety of symptoms can occur depending upon the site and severity of the arterial lesions.1 Peripheral arterial disease has been observed in 12% to 20% of the population, and the disorder affects men and women equally.2 Well-known risk factors such as smoking, diabetes, hyperlipidemia, hypertension, and family history increase the incidence of the disease.3 It is not entirely clear whether modification of these risk factors prevents the development of cardiovascular disease, but there is evidence that it may slow disease progression.4
The use of tobacco products appears to be one of the most important risk factors for the development of lower extremity atherosclerotic lesions. The amount of tobacco used is directly related to the extent of the disease, and cessation of tobacco use reduces the overall risk. Many other factors such as obesity, stress, and lack of exercise have been identified as contributing to the process.5 C-reactive protein (CRP) is a sensitive marker of cardiovascular inflammation, both systemically and locally. Slight increases in serum CRP levels are associated with an increased risk of damage to the vasculature, especially if these increases are accompanied by other risk factors such as advanced age, hypertension, hypercholesterolemia, obesity, elevated blood glucose levels, smoking, or a positive history of cardiovascular disease.6 Selvin and Erlinger7 reported that 95% of the 2174 participants aged 40 years and older in the National Health and Nutrition Examination Survey had at least one or more cardiovascular risk factors out of a selected 15 traditional risk factors.
Arteries can become damaged or obstructed as a result of atherosclerotic plaque, thromboemboli, chemical or mechanical trauma, infections or inflammatory processes, vasospastic disorders, and congenital malformations. A sudden arterial occlusion typically causes profound and often irreversible tissue ischemia and tissue death. However, when arterial occlusions develop gradually, there is less risk of sudden tissue death because collateral circulation has time to develop and the tissues can adapt to the decreased blood flow.
Arterial Embolism and Thrombosis
Arterial emboli arise most commonly from thrombi that originate in the chambers of the heart as a result of atrial fibrillation, myocardial infarction, infective endocarditis, or chronic heart failure. When these thrombi become detached, they are carried from the left side of the heart into the arterial system where they lodge in and obstruct an artery that is smaller than the embolus. Emboli may also originate at sites of advanced aortic atherosclerosis when the atheromatous plaques become irregular or ulcerate.
The symptoms associated with arterial emboli depend primarily on the size of the embolus, the organ or tissue involved, and the state of the collateral vessels. The immediate effect is cessation of distal blood flow. Secondary vasospasm can contribute to the ischemia. The embolus can fragment or break apart, resulting in occlusion of more distal vessels. Emboli tend to lodge at arterial bifurcations and areas narrowed by atherosclerosis. Cerebral, mesenteric, renal, and coronary arteries can be involved in addition to the large arteries of the extremities.
Arterial thrombosis can also acutely occlude an artery. Acute arterial thrombosis frequently occurs in patients with preexisting symptoms of chronic ischemia. Thrombi often originate where the arterial wall has become damaged, generally as a result of atherosclerosis, but they can also develop where flow is relatively stagnant, such as in an arterial aneurysm.
The manifestations of an acute thrombotic arterial occlusion are similar to those for embolic occlusion. The signs and symptoms associated with an ischemic limb are usually described in terms of the six “Ps”: pain, pallor, paresthesias, poikilothermia, pulselessness, and paralysis. Dr. Strandness also liked to add another “P” for the physician (although today he might use the term “provider” instead) who is the critical person in appreciating the significance of the presenting complaints. The symptoms of acute arterial occlusion in extremities with poor collateral flow are sudden severe pain and a gradual loss of sensory and motor function. Eventually, superficial veins may collapse because of decreased blood flow to the extremity. With severe ischemia, the part of the extremity distal to the occlusion is markedly colder and paler than the part proximal to the occlusion.
If the pain persists, it can be interpreted as a favorable sign, indicating that the nerves supplying the distal limb are still functioning. If the distal extremity is completely numb, the blood supply must be restored expeditiously if the nerves and other tissues are to survive. Dr. Strandness would emphasize to his students that the limb dies from the inside out—the nerves, then the muscles, and finally the skin. If it is suspected that the patient has limb-threatening ischemia, a prompt vascular consultation should be arranged, and diagnostic testing must be done without delaying the patient’s trip to the interventional suite or operating room for definitive treatment.
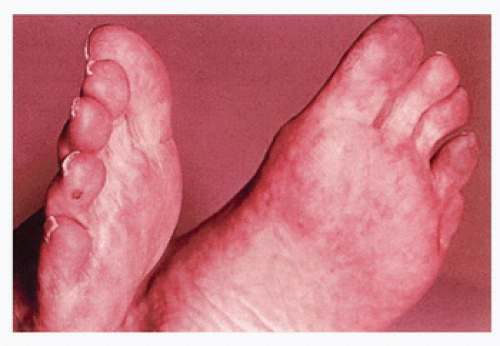
Pulse deficits are invariably present in patients with embolism or thrombosis of major extremity arteries, with one important exception: when there have been extensive micro-emboli to the distal foot or hand. In this circumstance, the major arteries are usually patent and serve as conduits for the small atheroemboli that typically arise from ulcerated plaques or areas of thrombosis at some point proximal to the foot and toes (Figs. 1.1 and 1.2). Major pulse deficits provide information only on the most proximal site of occlusion; they provide no information on the possible distal extension of the thrombotic process.
A detailed history with regard to symptoms before the acute event is important. If the patient gives a history of intermittent claudication, this is evidence that the patient has chronic arterial occlusive disease, with the current acute episode representing a thrombosis in the region of a preexisting high-grade stenosis. In the course of the physical examination, look for the presence of an aortic, femoral, or popliteal artery aneurysm that could be the source of emboli or thrombosis as a cause of acute limb ischemia.
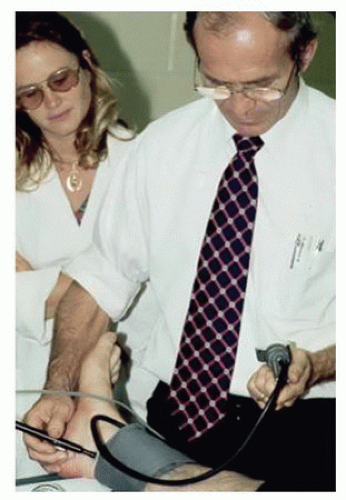
In patients who have palpable pulses to the level of the foot and appear to have microemboli as the cause of ischemia, listen for bruits from the level of the abdominal aorta to the popliteal fossa. Very few providers regularly listen over the superficial femoral and popliteal arteries for bruits, and Dr. Strandness would insist that this part of the physical examination be included in patients with suspected microemboli. The presence of a bruit indicates turbulent blood flow and a possible site of stenosis. Dr. Strandness told his residents that bruits are transmitted downstream from their site of origin, not upstream; “they go with the flow” was what he would say. Use a continuous wave (CW) Doppler to determine whether audible arterial flow is present in the tibial arteries. Dr. Strandness also considered ankle pressure measurement and calculation of the ankle-brachial index to be part of the routine vascular physical examination. He would often take
the time to perform this simple test himself, even when a vascular technologist was available (Fig. 1.3).
the time to perform this simple test himself, even when a vascular technologist was available (Fig. 1.3).
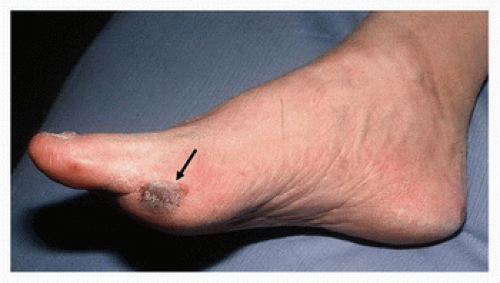 FIGURE 1.2. A focal area of skin infarction (black arrow) on the first metatarsal head caused by a small atheroembolus. |
The causes of acute ischemia in the upper extremity are similar to those found in the lower extremity, but some differences can be noted. Atherosclerosis of the arteries supplying the arm tends to involve only the innominate artery and the first part of the subclavian artery. Thus, acute ischemia secondary to thrombosis of an atherosclerotic plaque is very unusual. The principal cause of acute upper extremity ischemia is emboli, which most often arise from the heart. An infrequent source of emboli is a lesion in the subclavian artery at the thoracic outlet. Chronic compression of the subclavian artery at that site can lead to intimal damage with subsequent thrombosis and release of emboli. These events typically occur in younger patients who are in good health. Therefore, in any patient who suddenly develops acute ischemia of the hand, suspect damage to the subclavian artery from repeated compression.
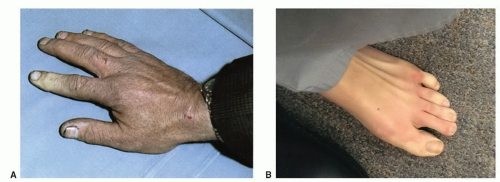
Other causes of acute hand ischemia are to be found among those disorders that include vasospasm, such as Raynaud’s syndrome, Buerger’s disease, scleroderma, and mixed connective tissue disorders, as discussed later in this chapter. Although vasospasm is usually a chronic problem, there are cases in which thrombosis of the digital and palmar arteries can lead to acute ischemia and gangrene. Dr. Strandness was known for his expertise in dealing with primary and secondary vasospastic disorders, and the most common underlying condition in his experience that was associated with this problem was scleroderma (Fig. 1.4). Many of the principles relating to the evaluation of acute ischemia of the hands are similar to those employed for the lower limb (see Chapters 12, 13, and 14).
Chronic Arterial Occlusion
The classic symptom of chronic peripheral arterial disease is intermittent claudication. This crampy muscular pain may be described by the patient as aching, fatigue, weakness, or even numbness in the extremity. However, the key feature of claudication is that it is consistently produced by the same intensity of leg exercise or activity and relieved with a few minutes of rest. It may gradually improve or get worse over months and years, but it is relatively constant from day to day. The symptoms are caused by the inability of the diseased arteries and collateral vessels to provide adequate blood flow to the tissues in the face of increased demands for nutrients and oxygen during exercise. The sites of the responsible arterial lesions can be deduced from the location of claudication because the symptoms occur in muscle groups distal to the occlusive disease. As the arterial lesions progress, patients may notice that their comfortable walking distance is shorter, or they may experience increased pain with ambulation. McLafferty and coworkers8 found that 50% to 70% of patients over 70 years of age with claudication perceived their discomfort to be a normal part of aging and did not report the symptoms to their providers.
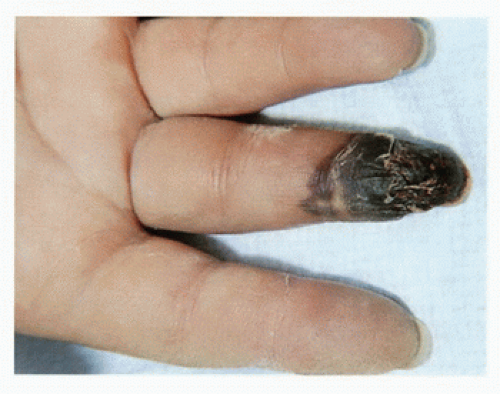 FIGURE 1.4. Patients with scleroderma may develop gangrene of the fingers. This is a result of thrombosis and occlusion of the digital arteries. |
Patients with unusual or atypical leg pain are commonly referred to a vascular specialist and the vascular laboratory for evaluation. These patients are often considered to have “pseudoclaudication” if no evidence of significant arterial occlusive disease is found. The most common nonvascular conditions responsible for such a presentation are neurospinal and orthopedic problems. The patient with pseudoclaudication frequently describes a walk-pain cycle that is not constant from day to day, for example, being able to walk a mile one day but limited to a few blocks the next day. In addition, the pain may be brought on by standing or sitting for prolonged periods of time, or the patient may have to lie down to obtain complete relief.
When arterial insufficiency becomes severe, the patient may have persistent pain in the distal foot and toes at rest. This “rest pain” indicates a critical state of ischemia, because it signifies that the tissue blood supply is inadequate at all times, not just during limb exercise. Rest pain is often worse at night and may interfere with sleep. Placing the limb in a dependent position typically reduces rest pain by producing a modest increase in distal limb blood flow. Changes resulting from chronic severe ischemia that can be noted on physical examination include loss of hair, brittle nails, dry or scaling skin, muscle atrophy, and ulcerations. Lower extremity edema may be apparent bilaterally or unilaterally and is usually caused by prolonged periods of dependency related to ischemic rest pain. Gangrenous changes or tissue necrosis appear after prolonged severe ischemia. In elderly patients who are inactive, gangrene may be the first sign of arterial occlusive disease (Fig. 1.5). These patients may have adjusted their lifestyle
to accommodate the limitations imposed by their disease and may never walk far enough to develop symptoms of claudication. Although their circulation is severely impaired, this is not apparent until ulceration develops.
to accommodate the limitations imposed by their disease and may never walk far enough to develop symptoms of claudication. Although their circulation is severely impaired, this is not apparent until ulceration develops.
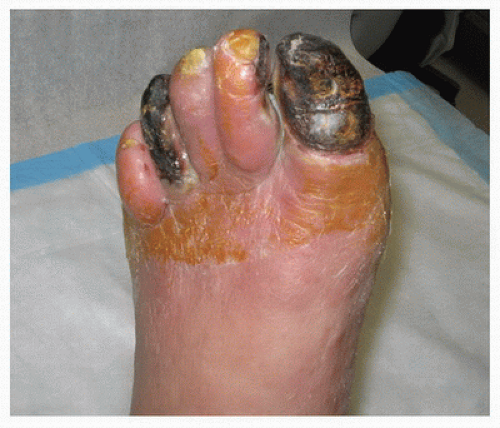 FIGURE 1.5. Necrosis or gangrene of several toes due to severe arterial occlusive disease in an elderly nonambulatory patient. |
Arterial occlusive disease can reduce or obliterate pulsations in the extremities. The level of pulse deficit is a good marker for the most proximal level of involvement. Palpate pulses bilaterally and simultaneously, if possible, comparing both sides for symmetry in rate, rhythm, and quality. Palpation of pulses is subjective, and the examiner may mistake their own pulse for that of the patient. To prevent this, the examiner should apply light pressure and avoid using only the index finger for palpation because this finger has the strongest arterial pulsation of all the fingers. The thumb also has a strong intrinsic pulsation and should not be used for pulse palpation.
Dr. Strandness was insistent that all vascular patients should have a thorough evaluation of their feet for trophic changes, especially for evidence of emboli or ulcerations. One of Dr. Strandness’ favorite stories was about a medical student who had been in to see a patient who had been followed for years in the clinic. The medical student gave his presentation and stated that the patient had normal lower extremity pulses bilaterally. Dr. Strandness did not say a word but went into the examination room with the medical student and pulled up the gentleman’s trousers, noting that the patient still had his socks and shoes on, and asked the student how he was able to palpate pulses through the shoes. The next thing he did was ask the patient to remove his below-knee prosthetic limb so he could assess the patient’s stump site. Dr. Strandness did not tell this story to make fun of the student (which the student clearly deserved), but rather to emphasize that all patients must have their socks and shoes removed for the physical examination.
Chronic arterial occlusive disease occurs less frequently in the upper extremities than in the lower extremities and causes less severe symptoms because the collateral circulation tends to be significantly better in the arms. The arms also have less muscle mass and are not subjected to the same heavy workload as the legs. As previously mentioned, upper extremity atherosclerotic lesions typically occur proximal to the origin of the vertebral artery, setting up the vertebral artery as a major contributor to collateral flow. When upper extremity arterial occlusive disease is symptomatic, the patient complains of arm fatigue and pain with exercise (arm claudication) and inability to hold or grasp objects (e.g., painting, combing hair, using hand tools, placing objects on shelves above the head, and occasionally difficulty driving). Dr. Strandness would stress to the vascular technologists that they should ask the patient about the specific activities that produced his or her symptoms and try to perform an evaluation under similar conditions. Many patients with symptomatic upper extremity arterial occlusive disease do not present with classic claudication. This condition may cause vertebrobasilar symptoms such as vertigo, ataxia, syncope, or bilateral visual changes. Significant findings on physical examination include coolness and pallor of the affected extremity, decreased capillary refill of the digits, and a difference in brachial systolic blood pressures of more than 10 to 15 mm Hg.
One of Dr. Strandness’ patients was a minister from a rural area who drove many miles daily visiting his parishioners. He initially presented to vascular surgery clinic for treatment of uncontrolled hypertension and renal artery stenosis. Once his renal artery disease had been treated and his blood pressure was under control, he began noticing that when driving and turning his head side to side he would become dizzy, and if he tilted his head, he developed severe vertigo. Duplex ultrasound evaluation showed that he had bilateral carotid artery disease and a subclavian artery occlusion. Physiologic noninvasive testing revealed that his upper extremity was stealing from his vertebrobasilar system when he drove long distances. What was most interesting about this patient was that when he was hypertensive, he appeared to have enough perfusion to his posterior circulation to be symptom free, but when his blood pressure was lowered to normal levels, he became symptomatic.
VASOSPASM
Patients who develop digital ischemia when their hands or feet are exposed to cold are often sent to the vascular laboratory for evaluation. This presentation is referred to as Raynaud’s syndrome and can be divided into primary Raynaud’s phenomenon and secondary Raynaud’s disease. Raynaud’s phenomenon is a common clinical disorder characterized by intermittent episodes of vasoconstriction of the small arteries of the hands and feet that cause dramatic color and temperature changes. Patients with Raynaud’s phenomenon have a young age of onset (usually <30 years), and the episodes are associated with minimal pain and typically involve all digits in a bilateral and symmetrical fashion. In this primary benign form, the palmar and digital arteries remain patent without any demonstrable structural abnormalities. Epidemiologic studies indicate that the prevalence of Raynaud’s phenomenon is between 6% and 20% in women and 3% and 13% in men. There is also a possible genetic susceptibility to the condition.9
By contrast, patients with Raynaud’s disease have asymmetrical digit involvement and intense pain related to an underlying pathologic condition (Fig. 1.6). This secondary form is more serious because it is associated with intrinsic occlusive lesions in the digital arteries and a long list of systemic diseases, of which the collagen-vascular diseases are the most common. Secondary Raynaud’s disease can also occur in the setting of arterial occlusions proximal to the wrist, hematologic syndromes, infectious diseases, and in patients with repetitive trauma from vibrating tools or anatomic abnormalities such as a cervical rib. Raynaud’s disease is a form of intermittent arteriolar vasoconstriction that results in coldness, pain, and pallor of the fingertips or toes. Symptoms may result from a defect in basal heat production that eventually decreases the ability of cutaneous vessels to dilate. Episodes may be triggered by emotional factors or unusual sensitivity to cold.
The classic clinical picture consists of three phases and begins with pallor of the fingers or toes brought on by sudden
intense vasoconstriction. The skin then becomes bluish (cyanotic) due to pooling of deoxygenated blood in the tissues. The final phase occurs as vasospasm resolves and the small arteries vasodilate, producing a hyperemic state. This results in a reddish color (rubor) as oxygenated blood returns to the digits. The characteristic sequence of color changes in cold sensitivity of the Raynaud type is described as “pallor, cyanosis, and rubor” or “white, blue, and red.” Numbness, tingling, and burning pain may occur as the colors change. The primary role of the vascular laboratory is to determine the anatomic status of the circulation to the digits and assess their response to cold exposure (see Chapter 14). The physical examination is usually not helpful unless digit ulceration is noted.
intense vasoconstriction. The skin then becomes bluish (cyanotic) due to pooling of deoxygenated blood in the tissues. The final phase occurs as vasospasm resolves and the small arteries vasodilate, producing a hyperemic state. This results in a reddish color (rubor) as oxygenated blood returns to the digits. The characteristic sequence of color changes in cold sensitivity of the Raynaud type is described as “pallor, cyanosis, and rubor” or “white, blue, and red.” Numbness, tingling, and burning pain may occur as the colors change. The primary role of the vascular laboratory is to determine the anatomic status of the circulation to the digits and assess their response to cold exposure (see Chapter 14). The physical examination is usually not helpful unless digit ulceration is noted.
Buerger’s disease is one of the systemic disorders associated with the development of cold sensitivity and vasospasm. It is regarded as an autoimmune vasculitis and occurs most often in men between 20 and 35 years of age. Considerable evidence indicates that heavy smoking or chewing of tobacco is a causative or aggravating factor.10 The vasospastic manifestations of Buerger’s disease are generally bilateral and symmetrical, with focal occlusive lesions in the small arteries. Pain is the outstanding symptom, and it is often aggravated by nicotine, cold exposure, or emotional disturbances.
ABDOMINAL AORTIC ANEURYSM
An aneurysm is a permanent localized dilatation of an artery. Symptoms are variable and depend on how rapidly the artery dilates and how the pulsating mass affects surrounding structures. Many patients are asymptomatic, and the aneurysm is found incidentally during a workup for some other problem. A history of smoking is the risk factor most strongly associated with an abdominal aortic aneurysm (AAA), but other factors such as having a first-degree relative with an AAA and having atherosclerosis are also independent risk factors.11 Most aneurysms in the abdominal aorta occur below the level of the renal arteries. AAAs are more common among Caucasians, affecting men four to six times more often than women.12 Some patients complain that they can feel their heart beating in their abdomen when lying down, or they may describe a “throbbing” feeling in their abdomen.
The most important diagnostic sign of an AAA is a palpable pulsatile mass in the epigastric area or upper abdomen. A systolic bruit may be auscultated over the mass. The physical examination should include palpation of the abdominal aorta and the iliac, femoral, and popliteal arteries to determine whether there is a bounding pulse. As has been previously mentioned, if an AAA is present or suspected, evaluate the toes for evidence of embolization. When a small (3 to 4 cm in diameter) AAA is identified, serial ultrasound follow-up is often recommended at 6- to 12-month intervals until the aneurysm reaches a size at which intervention is justified—typically 5 to 6 cm in diameter. An aneurysm that shows rapid growth or becomes symptomatic warrants an urgent vascular consultation.
Aneurysms may also arise in the peripheral arteries. The most frequent site for peripheral aneurysms is the popliteal artery, but other arterial sites such as the subclavian, renal, or common femoral arteries can also be involved. Between 50% and 60% of popliteal aneurysms are bilateral and may be associated with AAAs. If a bounding popliteal pulse is palpated, perform a duplex ultrasound to rule out a popliteal artery aneurysm. Aortic and peripheral artery aneurysms are discussed further in Chapter 15, and point-of-care screening for AAA is covered in Chapter 27.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree