Follow-Up After Carotid Endarterectomy and Stenting
Robert M. Zwolak
R. Eugene Zierler
Carotid endarterectomy (CEA) and carotid artery stenting (CAS) are procedures performed to prevent atheroembolic stroke. CEA has been a standard therapy since the early 1970s, whereas CAS was added by Medicare in 2005 as a covered treatment modality for symptomatic patients considered to be at high risk for CEA. The decision to cover CAS was made following publication of a randomized trial comparing CAS to CEA in high-risk subgroups.1 Duplex ultrasound has played a major role in both of these therapies, initially for identifying appropriate treatment candidates and subsequently for following patients after their procedures. This chapter focuses on follow-up after carotid interventions. The general approach to the duplex evaluation of extracranial carotid artery disease is discussed in Chapter 8.
The utilization of CEA increased dramatically from approximately 15,000 cases in 1971 to 107,000 in 1985.2 However, the total number of CEA cases decreased after that time, purportedly due to uncertainty regarding the specific indications and concerns over operative risk. The randomized clinical trials published in the 1990s compared the results of CEA to the medical management available at that time and helped to clarify the indications for CEA in both symptomatic and asymptomatic patients.3,4 In the late 1990s, CAS was introduced as a less invasive alternative to CEA, and utilization of CAS increased from 3% of all carotid revascularization procedures in 1998 to 13% in 2008.5 Even with this increase in CAS, the total number of carotid revascularization procedures decreased over this time period as a result of a larger decrease in the number of CEA operations performed.6 The outcomes and safety of both CEA and CAS were compared in the Carotid Revascularization Endarterectomy versus Stenting Trial, which was reported in 2010.7 There was no significant difference between CEA and CAS in the incidence of the primary composite end point of stroke, myocardial infarction, or death in the periprocedural period at 4.5% and 5.2%, respectively. However, there was a difference in the rates of the separate end points, with a lower incidence of stroke in the CEA group and a lower incidence of MI in the CAS group.
Noninvasive evaluation of cerebrovascular disease was the first widespread application of duplex ultrasound scanning.8,9 As this technique evolved and improved, duplex ultrasound was shown to be sufficiently accurate to serve as the exclusive preoperative testing modality prior to CEA in centers where sonographers and interpreting physicians focused on providing the highest-quality examination possible.10,11 The safety of CEA has been well established. The largest multi-center prospective trial of CEA in asymptomatic patients in the United States published in 1995 demonstrated a 2.3% incidence of the combined end point of stroke plus death at 30 days.4 Fifteen years later, the Society for Vascular Surgery Vascular Registry identified a 2.0% incidence of the combined end point of stroke plus death plus myocardial infarction at 30 days in asymptomatic patients.12 The low morbidity and mortality after CEA is due to a wide range of factors, including careful patient selection, high-quality anesthesia, close attention to technical surgical details, and tightly controlled postoperative care.
Restenosis after CEA and CAS appears to occur infrequently in contemporary series, and duplex ultrasound has been advocated as an appropriate means to detect this complication and help determine the need for reintervention. In the Carotid Revascularization Endarterectomy versus Stenting Trial, restenosis was defined as a diameter reduction of 70% or greater as indicated by a peak systolic velocity (PSV) of 300 cm/s or more.13 Follow-up by serial duplex scanning for 2 years showed no significant difference between the CEA and CAS groups for a combined end point of restenosis or occlusion with an incidence of 6.3% and 6.0%, respectively. The Kaplan-Meier estimate for the frequency of the combined end point at 4 years was 6.2% for CEA and 6.7% for CAS.
DUPLEX AFTER CAROTID ENDARTERECTOMY
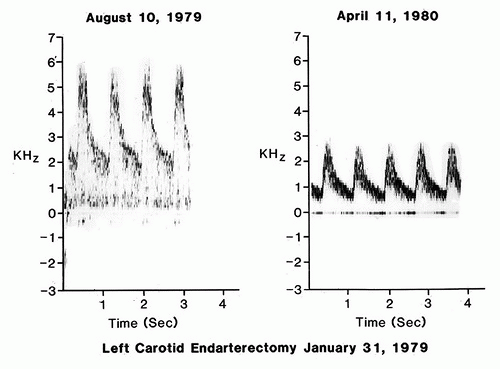
Early reports from authors including Zierler and coworkers,14 Baker and colleagues,15 Healy and associates,16 and others described the use of duplex ultrasound for serial follow-up after CEA, and the range of reported residual or recurrent stenosis varied widely from 4% to 21%. The initial series described by Zierler and coworkers included 76 patients undergoing 89 CEA operations who were followed by duplex scanning for a mean of only 16 months.14 Duplex findings indicating greater than 50% restenosis (based on velocity criteria for native atherosclerotic lesions) were observed in 32 of the 89 internal carotid arteries; however, some of these early postoperative lesions regressed on serial follow-up, and the
overall incidence of persistent restenosis was 19% (Fig. 10.1). Healy and associates followed 301 patients after CEA, with most of the cases having primary arteriotomy closure without patch angioplasty.9 By life-table analysis at 7 years, 31% of the patients developed a restenosis of greater than 50%, but 10% of these subsequently regressed. Despite a net restenosis prevalence of 21%, only 3% of patients suffered a stroke during follow-up, and this incidence of postprocedure stroke was no different from that in patients without early restenosis. The authors concluded that a conservative approach could be taken toward restenosis.16
overall incidence of persistent restenosis was 19% (Fig. 10.1). Healy and associates followed 301 patients after CEA, with most of the cases having primary arteriotomy closure without patch angioplasty.9 By life-table analysis at 7 years, 31% of the patients developed a restenosis of greater than 50%, but 10% of these subsequently regressed. Despite a net restenosis prevalence of 21%, only 3% of patients suffered a stroke during follow-up, and this incidence of postprocedure stroke was no different from that in patients without early restenosis. The authors concluded that a conservative approach could be taken toward restenosis.16
Bernstein and coworkers followed 566 patients after CEA and found a 50% or greater restenosis rate at 10 years (life-table analysis) of 14.5% in women and 7.7% in men (P < 0.003).17 Curiously, these authors found early restenosis to be protective—patients with restenosis were statistically less likely to have late symptoms, stroke, or early death than were patients with normal postoperative duplex scans. Mackey and colleagues followed 348 patients after CEA and observed a 16% rate of 50% or greater restenosis at a mean 4-year follow-up.18 Like Bernstein and coworkers, these authors concluded that the stroke risk related to early restenosis was low. Without performing a formal cost-benefit analysis, this group suggested that routine duplex follow-up after CEA was difficult to justify.
In 1996, Ricotta and DeWeese published a single-center retrospective analysis focusing on progression of contralateral carotid disease in patients undergoing CEA.19 They reviewed 562 patients who underwent 660 operations over a 13-year period. Approximately 30% of these patients had greater than 50% diameter stenosis in the contralateral carotid artery at initial presentation, and about half of these underwent CEA during early follow-up. For patients with less than 50% contralateral carotid stenosis at presentation, they documented a 10% progression rate to greater than 50% stenosis at 4.5 years mean follow-up. They concluded that patients with minimal contralateral carotid stenosis and a widely patent ipsilateral carotid artery after CEA could be followed using duplex ultrasound at intervals of up to 2 years.
A single-center report by Roth and associates in 1999 assessed the value of duplex scanning during and after CEA.20 Duplex scans were performed intraoperatively and after operation at 3- to 6-month intervals for a mean follow-up of 27.4 months. Threshold criteria for a 50% to 74% stenosis included PSV greater than 125 cm/s, end-diastolic velocity (EDV) less than 125 cm/s, and an internal carotid artery to common carotid artery (ICA/CCA) PSV ratio greater than 2.5. Threshold criteria to diagnose a 75% to 99% stenosis included PSV greater than 300 cm/s, EDV greater than 125 cm/s, and ICA/CCA PSV ratio greater than 4.0. During follow-up, six arteries (2.7%) developed restenosis greater than 50%, but only one (<1%) developed restenosis greater than 75%, and this latter patient underwent reoperation. Similar to the observations of Ricotta and DeWeese, Roth and associates found a higher rate of progression to severe disease on the side contralateral to the CEA, with 12.7% of patients showing an increase in carotid stenosis by at least one duplex stenosis category during follow-up. Overall, these studies from the 1990s suggested that serial duplex scanning after CEA is more justified for surveillance of contralateral untreated disease than for ipsilateral recurrent stenosis.
These studies form the basis for current duplex follow-up algorithms after CEA. As a related observation, in view of the low morbidity associated with restenosis after CEA documented in these reports, it is noteworthy that a large number of carotid stents have been placed for recurrent stenosis. For instance, 34% of the 480 patients undergoing CAS in the Boston Scientific EPI: A Carotid Stenting Trial for High-Risk Surgical Patients (BEACH) trial of the Boston Scientific WALLSTENT were performed for restenosis after CEA.21
The technique of placing a patch for closure of the arteriotomy during CEA (patch angioplasty) likely has a significant impact on the development of restenosis. In a 1987 report, Ouriel and Green found that patch angioplasty was associated with a lower rate of early restenosis.22 In 1994, Myers and coworkers published a single-center prospective, randomized, controlled trial wherein they were unable to demonstrate superior long-term results with vein patch closure as compared with primary arteriotomy closure during CEA.23 However, by 2004, Bond and colleagues were able to identify seven randomized, controlled trials involving 1281 operations comparing routine patch closure with primary closure during CEA, and patch angioplasty was associated with a statistically significant reduction in risk for perioperative and late stroke.24 The strength of these data is such that the National Quality Forum has passed a quality measure supporting patch closure for conventional noneversion CEA.25
Ultrasound Findings
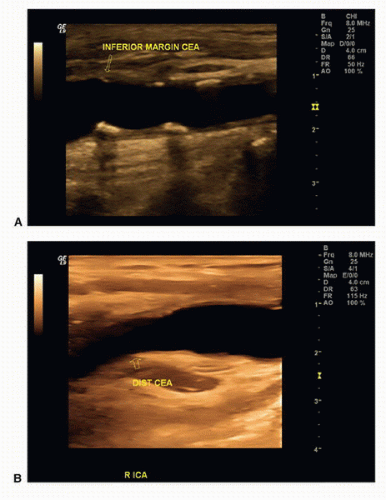
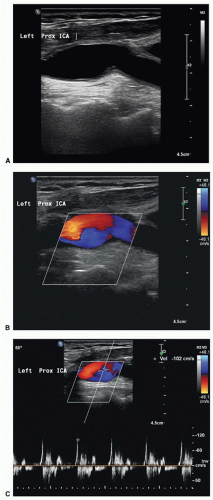
Duplex examinations performed on patients in the weeks or months after CEA will most often demonstrate a widely patent endarterectomy site (Fig. 10.2). The proximal initiation point and the distal end point of the endarterectomized segment are usually visible. The location of the proximal “shelf” is determined by surgical technique and the severity of disease in the CCA. The caliber of the lumen and its shape depend on whether primary arteriotomy closure, eversion endarterectomy, or conventional endarterectomy with patch angioplasty closure has been performed. B-mode and color flow imaging typically demonstrate wide patency, although there may be color flow swirling (flow separation) at the CEA site if a patch has been placed (Fig. 10.3). Minor flow disturbances may be seen at the endarterectomy distal end point, but ordinarily, there should be no significantly elevated velocities or extreme flow disturbance. Modest flow disturbances seen early after CEA may disappear on subsequent studies owing to natural vessel remodeling.
The endarterectomy suture line may be visible on B-mode imaging. Sutures appear as small, bright, regularly spaced echoes along the near wall of the vessel, as shown in Figure 10.4. Some surgeons apply stainless steel clips on small arteries and veins during the operation, and these small but highly echogenic objects may cause acoustic shadows that obscure portions of the underlying vessels. Flaps of tissue may be seen in the endarterectomized portion of the artery on intraoperative imaging (Fig. 10.5). Most large flaps that cause flow disturbances should be treated. The actual natural history of very small flaps in the endarterectomized segment remains poorly defined. Intraoperative assessment of CEA is discussed in Chapter 30.
Ultrasound visualization of a patch after CEA depends on the type of patch material used and the time interval after surgery. Polytetrafluoroethylene (PTFE) is often seen as a bright double-line interface on the near wall of the artery. Dacron patches are thick and also echogenic. If scanned during the first one or two days after surgery, the small amount of air trapped within the PTFE and Dacron material may cause acoustic shadowing and difficulty visualizing the vessel lumen. Autogenous saphenous vein and bovine pericardial patches may not be distinguishable from the native tissue, even in the early postoperative period, but the patched segment can be recognized by a bulging appearance over the endarterectomy site and the possible visualization of adjacent sutures (Figs. 10.3 and 10.4).
Restenosis that occurs in the endarterectomized segment during the first postoperative year is typically due to intimal hyperplasia. This is substantially different tissue than typical atherosclerotic plaque. Lacking any significant calcium deposits, the intimal hyperplastic lesion tends to be echolucent and commonly forms a long uniform narrowing (Fig. 10.6). Peak velocities may be extremely elevated throughout the stenotic segment. Restenosis that occurs 5 to 10 years postoperatively usually represents recurrent atherosclerotic plaque, particularly in an individual whose risk factors have not been successfully treated.
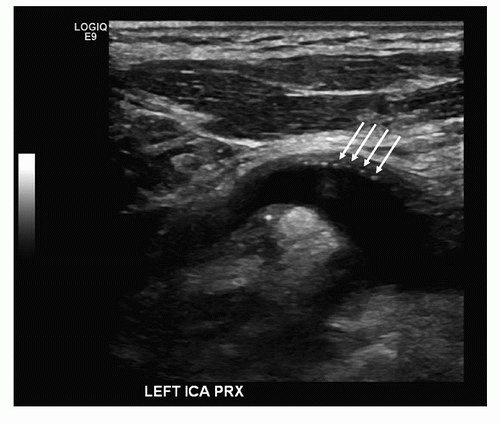 FIGURE 10.4. B-mode image of a patched internal carotid artery (ICA) showing sutures as bright “dots” on the near wall of the vessel (arrows). |
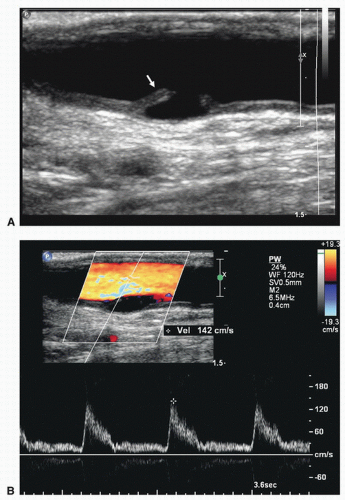 FIGURE 10.5. Intraoperative duplex assessment after carotid endarterectomy. A, Longitudinal B-mode image of a flap in the internal carotid artery (arrow). B, Color flow image of the same flap and a Doppler spectral waveform showing no significant flow disturbance associated with this finding.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|