The Cardiac Action Potential
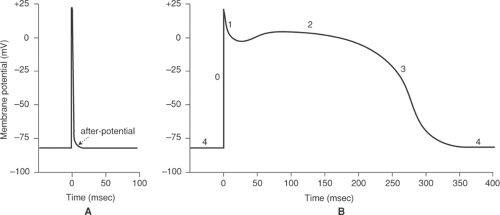
The cardiac action potential, which is generated by the orchestrated opening and closing of the ion channels described in Chapter 13, is much more complex than the action potentials in nerves and skeletal muscle, where depolarization lasts only a few milliseconds (Fig. 14-1A). In the heart, action potentials last several hundred milliseconds, consist of several phases, and vary in characteristics from region to region. Purkinje fiber action potentials, for example, are large, rapidly rising, last over 300 ms, and include five distinct phases (Fig. 14-1B). These include a large rapid upstroke (phase 0) that is followed by two phases, transient repolarization (phase 1) and a plateau (phase 2), that do not have clear counterparts in nerve and skeletal muscle. Purkinje fiber action potentials end when repolarization (phase 3) returns membrane potential to its resting level during diastole (phase 4), after which these myocytes often exhibit spontaneous depolarizations (pacemaker activity). Action potentials in the working cells of the ventricles are similar to those of Purkinje cells, except that they are somewhat smaller and lack pacemaker activity; atrial action potentials resemble those of the ventricles except that they are briefer, while action potentials in the SA and AV nodes are much smaller and lack a plateau.
During each action potential, a small amount of sodium enters working cardiac myocytes and the cells of the His-Purkinje system, while potassium is lost. It is a frequent misconception that the concentration gradients for sodium and potassium across the plasma membrane are dissipated during each action potential. However, a single depolarization causes only a very small change in the chemical composition of the cardiac cell, as evidenced by the large number of action potentials that can be generated after the Na-K ATPase is poisoned. Although prolonged rapid stimulation does cause cells to gain a measurable amount of sodium, the increase is small; for example, stimulation of a sheep Purkinje fiber for 3 min at 1 Hz has been estimated to increase intracellular sodium activity by only 1.9 mM (Cohen et al., 1982).
Resting Potential
Resting potential in myocardial cells is related to the electrochemical gradient for potassium across the plasma membrane, which is permeable to potassium but impermeable to the anionic proteins and phosphate-containing compounds found in the cytosol; this establishes a Donnan equilibrium where [K+]i is higher than [K+]o and the cytosol is negatively charged (see Chapter 13). In this Donnan equilibrium, the tendency for potassium to move down its concentration gradient out of the cell is balanced by the negative intracellular resting potential that favors potassium influx; in other words, the tendency of the potassium concentration gradient to cause potassium to leave the cell is countered by an electrical gradient that causes potassium to enter the cell.
The Nernst and Goldman–Hodgkin–Katz Equations
The plasma membrane in resting cardiac myocytes is not perfectly selective for potassium. There is, for example, a small permeability to sodium which, because of the higher sodium concentration outside the cell (Table 14-1) favors a membrane potential opposite in polarity to that associated with the potassium gradient. The membrane potential that results from the
distributions of these and other ions is described by the Nernst and Goldman–Hodgkin–Katz equations, which define the equilibrium potential (where there would be no net flux of ions) established by all of the ion concentration gradients across the plasma membrane. The Nernst equation describes the equilibrium potential established by a single ion, while the Goldman–Hodgkin–Katz equation describes the potential across a membrane that is permeable to several ions.
distributions of these and other ions is described by the Nernst and Goldman–Hodgkin–Katz equations, which define the equilibrium potential (where there would be no net flux of ions) established by all of the ion concentration gradients across the plasma membrane. The Nernst equation describes the equilibrium potential established by a single ion, while the Goldman–Hodgkin–Katz equation describes the potential across a membrane that is permeable to several ions.
Table 14-1 Ion Activities Inside and Outside Mammalian Myocytes | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
The Nernst Equation
The membrane potential established by a concentration difference for a single ion across a semi-permeable membrane is described by the Nernst equation:

where Em is the membrane potential, R the gas constant, T the absolute temperature, z the valence of the ion, F the Faraday constant, P the permeability to the ion, and ao and ai the activities of the ion outside and inside the membrane, respectively. The Nernst equations generated at 37°C when alkali metal ions such as sodium and potassium, for which z = 1, are distributed across a freely permeable membrane (P = 1) can be written for ordinary (base 10) logarithms as:

Equation 14-2 states that a tenfold difference in the activity of a monovalent cation, where ao/ai = 10, generates a potential difference of +61.5 mV. If the concentration gradient is reversed, and ao/ai = 0.1, Em is -61.5 mV.
In a resting cardiac myocyte, where [K+]o is ∼5.4 mM and [K+]i is ∼120 mM, the corresponding potassium ion activities are approximately 4 and 100 mM (Table 14-1). If the plasma membrane in resting cardiac myocytes was freely permeable to potassium, and impermeable to all other ions for which a concentration gradient exists between the inside and outside of the cell, Em would equal EK:
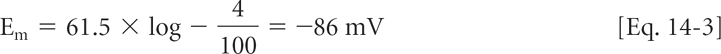
In most regions of the heart, resting potential is close to that predicted by the Nernst equation for potassium.
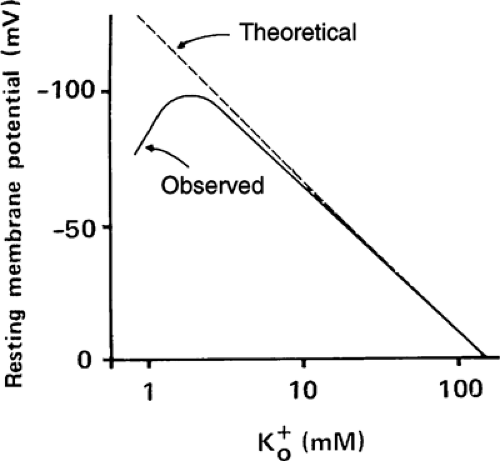
Changes in extracellular potassium concentration ([K+]o) have two effects on resting potential. The first is due simply to changes in the Nernst potential for potassium; according to Equation 14-3, increased [K+]o causes depolarization and reduced [K+]o hyperpolarizes the plasma membrane. The second occurs because very low [K+]o (<3 mM) reduces potassium permeability (PK). As a result of this hypokalemia-induced fall in PK at low [K+]o, resting potential does not follow the Nernst equation for potassium (Fig. 14-2) because the increased influence of the other ions, notably sodium, shifts membrane potential away from that predicted by the Nernst equation.
The Goldman–Hodgkin–Katz Equation
More accurate estimates of membrane potential are calculated by the Goldman-Hodgkin-Katz equation, which takes into account the permeabilities and activities of all ion species for which there is a gradient across the membrane. For a membrane permeable to sodium and chloride, as well as to potassium, this equation is
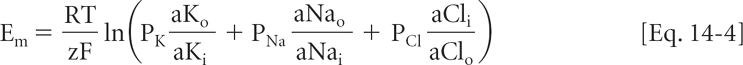
Equation 14-4 highlights the fact that the contribution of any ion to membrane potential is determined both by the activity gradient across the membrane and the permeability of the membrane to that ion. The contribution of any ion to Em disappears if there is no activity gradient, or if permeability to the ion becomes zero.
Some Features of the Cardiac Action Potential
Threshold
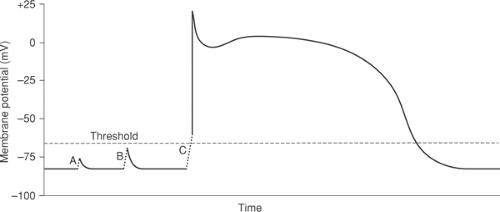
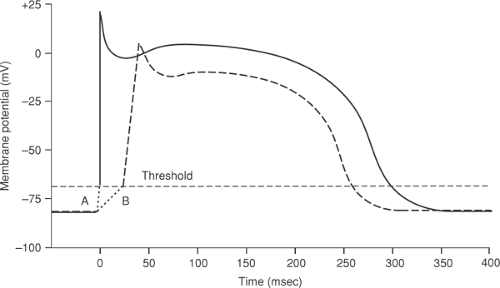
In order for an electrical stimulus to initiate an action potential, depolarization must reach a threshold. Smaller (subthreshold) depolarizations cause only local responses because they do not open enough depolarizing channels to initiate a regenerative action potential (Fig. 14-3A and B). The threshold is the lowest membrane potential at which opening of enough sodium channels—or calcium channels in nodal cells—is able to initiate the sequence of channel openings needed to generate a propagated action potential. The latter is often called a regenerative response because, once threshold is reached, the extent of the subsequent depolarization becomes independent of the initial depolarizing stimulus (Fig. 14-3C).
Although small depolarizations that fail to reach threshold do not initiate action potentials, they can have important effects on the responses to subsequent depolarizing currents. Most important is the ability of subthreshold depolarizations to cause transient inactivation of sodium
channels by closing their h gates, which decreases both the rate and extent of depolarization in subsequent action potentials.
channels by closing their h gates, which decreases both the rate and extent of depolarization in subsequent action potentials.
Rate of Rise and Amplitude of the Action Potential
The rate of depolarization (dV/dt) during an action potential upstroke (phase 0) is determined by the rate at which cations ions enter the cytosol. In cells whose action potentials depend on sodium channel opening, initial depolarizing currents develop rapidly, are very large, and last only for a short time; for this reason, sodium currents are often referred to as fast inward currents. The action potentials in the SA and AV nodes have a lower amplitude and slower rate of rise because depolarization depends on the slower opening of smaller (lower conductance) calcium channels.
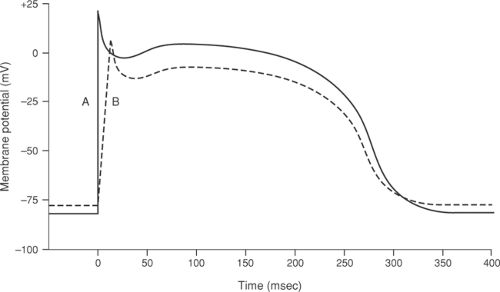
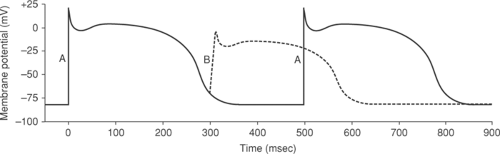
Membrane potential immediately prior to stimulation is an important determinant of sodium channel opening, and so determines the amplitude and rate of rise of the action potential. In a cardiac myocyte with a normal resting potential of between -80 and -90 mV, depolarizing stimuli that exceed threshold generate large action potentials with a rapid upstroke, whereas if the cell is partially depolarized prior to stimulation, the same depolarizing stimulus produces a smaller, more slowly rising action potential (Fig. 14-4) because the prior subthreshold depolarization inactivates some of the sodium channels. Sodium channel activation also depends on the rate at which threshold is approached. When threshold is reached quickly, sodium conductance increases rapidly which causes action potential upstroke to be rapid and the amplitude to be high (Fig. 14-5A), whereas if a longer time elapses before threshold is reached, some of the sodium channels become inactivated, which generates a lower amplitude action potential that rises more slowly (Fig. 14-5B). These manifestations of voltage-dependent inactivation of sodium channel opening are important clinically because reduced action potential amplitude and rate of rise provides a substrate for arrhythmias by slowing impulse conduction (see Chapter 16).
The Plateau
When cardiac action potentials were first observed to have a plateau, it was not known whether membrane potential had stabilized because current flow had ceased, or because currents continued to flow into and out of these cells, but had become equal. The answer was provided by Weidmann (1951), who found that unlike skeletal muscle and nerve, where membrane resistance increases sharply a few milliseconds after depolarization, resistance in cardiac muscle remains low early during the plateau; this means that currents are flowing in both directions across the plasma membrane. Subsequent research has shown that, during the plateau, outward potassium currents are balanced by an inward calcium current.
Refractory Periods
Like resting depolarization (Fig. 14-4) and a slow rate of activation (Fig. 14-5), stimulation of a ventricular myocyte before it has recovered from a preceding depolarization results in small, slowly rising action potential (Fig. 14-6). The mechanisms are similar; all inactivate the sodium channels normally responsible for depolarization. Both resting depolarization and slow activation reduce the rate of rise and amplitude of the action potential as a result of depolarization-induced channel inactivation, while the slowly rising, small action potentials seen when
activation is premature reflect the slow time course of channel reactivation. In cardiac sodium channels, complete recovery of the h gates can require more than 100 ms after membrane potential has returned to its resting level.
activation is premature reflect the slow time course of channel reactivation. In cardiac sodium channels, complete recovery of the h gates can require more than 100 ms after membrane potential has returned to its resting level.
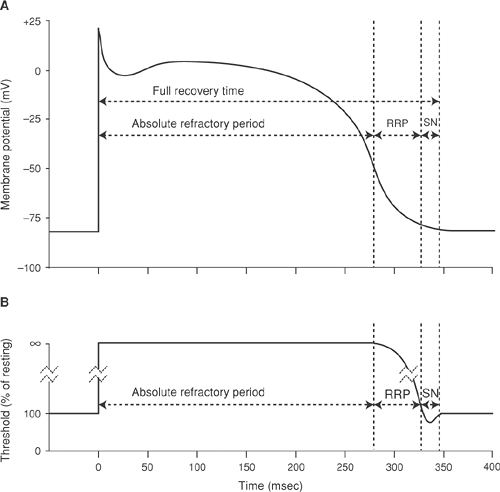
The period of depressed excitability that begins when depolarization closes the h gates of sodium channels, called the refractory period, is traditionally divided into two phases (Fig. 14-7). The first is the absolute refractory period, when no stimulus, whatever its magnitude, can evoke
a propagated response. This is followed by the relative refractory period, when only stimuli that exceed the normal threshold can initiate a propagated response. The interval between the onset of depolarization and the return of normal excitability, sometimes called the full-recovery time, encompasses the effective and relative refractory periods, as well as the supernormal period described below. The long refractory period of myocardial cells is due both to the prolonged plateau of the action potential, which delays the return of membrane potential to the resting level at which the sodium channels reactivate, and the slow rate of channel recovery after the membrane has repolarized.
a propagated response. This is followed by the relative refractory period, when only stimuli that exceed the normal threshold can initiate a propagated response. The interval between the onset of depolarization and the return of normal excitability, sometimes called the full-recovery time, encompasses the effective and relative refractory periods, as well as the supernormal period described below. The long refractory period of myocardial cells is due both to the prolonged plateau of the action potential, which delays the return of membrane potential to the resting level at which the sodium channels reactivate, and the slow rate of channel recovery after the membrane has repolarized.
Action potentials evoked during the heart’s relative refractory period depolarize slowly and are of low amplitude (Fig. 14-7), so that they conduct slowly (see Chapter 16). Stimuli that reach the heart shortly after refractoriness has ended, even after threshold has returned to normal, may still initiate small, slowly conducting action potentials because of the slow reactivation of cardiac sodium channels.
Supernormality and Vulnerability
A supernormal period, sometimes seen at the end of the relative refractory period (Fig. 14-7), is characterized by the ability of stimuli smaller that those needed to reach the normal threshold to produce propagated action potentials. Although threshold is reduced during supernormality, the action potentials generated during this period are of low amplitude. This apparent discrepancy reflects the fact that although threshold is low, not all sodium channels have recovered. Supernormality is well documented in isolated His-Purkinje cells, but is probably absent in the AV node, atria, and ventricles (Spear and Moore, 1980). Evidence for this phenomenon can occasionally be identified on the clinical electrocardiogram (see Chapter 16).
The supernormal phase occurs at approximately the same time as the vulnerable period, but vulnerability and supernormality are different phenomena. Supernormality is a lowered threshold, whereas vulnerability describes an increased susceptibility to ventricular fibrillation caused by heterogeneities in repolarization (see Chapter 16).
Rectification
Ion channels can exhibit a property called rectification, which is the ability of changing membrane potential to modify channel conductance—the ability to carry current (Fig. 14-8). In an unrectified (Ohmic) current, resistance (R) is independent of membrane potential (E), so that the current (I) is simply an inverse linear function of voltage

Rectification occurs when changing membrane voltage either increases or decreases channel conductance.
Most of the potassium currents carried by voltage-gated potassium channels across biological membranes are rectified (Fig. 14-8). Outward rectification occurs when depolarization increases potassium conductance. As a result, outward rectification increases the flow of repolarizing potassium current when the membrane depolarizes which, by helping depolarized cells to repolarize, favors the return of membrane potential to the resting level. Outward rectification can therefore be viewed as a “true” rectification because it “puts things right” by helping to end the
action potential. Inward rectification, which occurs when depolarization decreases potassium conductance, has the opposite effect because it responds to depolarization by decreasing the flow of outward current. Inward rectifier potassium currents therefore tend to maintain the membrane in a depolarized state. Because inward rectification was once considered to be an anomaly, it was also called “anomalous” rectification. These differences in rectification reflect the different molecular structures of the α-subunits of the potassium channels that carry outward and inward rectifying currents (see Chapter 13).
action potential. Inward rectification, which occurs when depolarization decreases potassium conductance, has the opposite effect because it responds to depolarization by decreasing the flow of outward current. Inward rectifier potassium currents therefore tend to maintain the membrane in a depolarized state. Because inward rectification was once considered to be an anomaly, it was also called “anomalous” rectification. These differences in rectification reflect the different molecular structures of the α-subunits of the potassium channels that carry outward and inward rectifying currents (see Chapter 13).
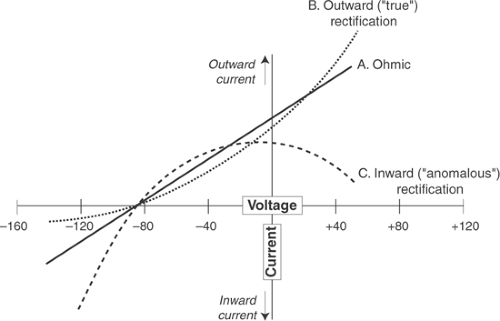 Fig. 14-8: Current–voltage relationships showing rectification of potassium currents. The effects of changing membrane potential (abscissa) on ionic currents (ordinate) are shown for three different types of potassium channel; inward currents are downward, outward currents are upward. All of the potassium currents are zero at -86 mV, which is the equilibrium potential for potassium. An unrectified (Ohmic) current (solid line A) is a linear function of membrane voltage because channel conductance is independent of membrane voltage. Outward (“true”) rectification is seen when depolarization increases potassium conductance (dotted line B), which allows depolarization to increase the repolarizing potassium currents that return membrane potential to the resting level. Inward (“anomalous”) rectification occurs when depolarization decreases potassium conductance (dashed line C), which tends to maintain membrane potential in the depolarized state by decreasing repolarizing potassium currents.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|