Fig. 1.1
Scheme of the sympathetic and parasympathetic nervous system (see text for description)
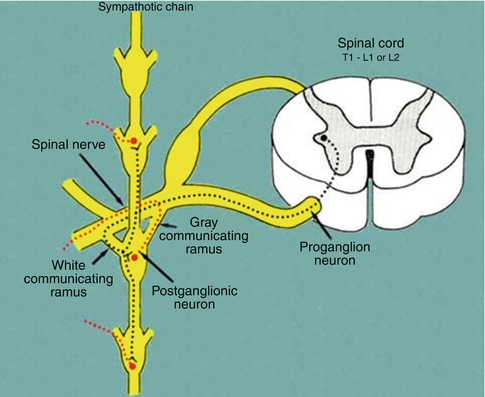
Fig. 1.2
Nerve connections between the spinal cord, spinal nerves, sympathetic nervous chain, and peripheral sympathetic nerves
The SNC runs on either side of the anterior face of vertebral bodies, extending from the cranium base to the coccyx and includes paravertebral ganglia along its course, specifically, 3 cervical ganglia, 11 or 12 thoracic ganglia, and 5 lumbar, 4 sacral, and 1 coccyx ganglia, which are interconnected by intermediate cords and contain the bodies of postganglionic sympathetic neurons (Fig. 1.1).
Preganglionic axons coming from a given spinal segment and nerve may terminate onto a postganglionic neuron located in the corresponding segmental ganglion or may instead travel either up or down along the SNC, synapsing onto a postganglionic neuron located in another SNC paravertebral ganglion.
Some sympathetic preganglionic fibers do not synapse in paravertebral ganglia but from SNC arrive to some specific prevertebral ganglia in the lumbar region (celiac, aorticorenal, and mesenteric ganglia) or ganglia located very proximal to target organs (peripheral ganglia). Finally, the SNC also sends out peripheral nerves that reach the target organs following the course of vessels (perivascular branches).
Postganglionic neurons are small nonmyelinated C fibers (0.3–1.2 μm) that reach the target organs by various ways. Some achieve somatic structures of the body (skin, muscles, bones) through spinal nerves that they reach from the SNC by gray rami communicantes (Fig. 1.2); some from cervical and high thoracic ganglia directly achieve cranial structures through peripheral nervous branches; similarly, visceral thoracic and abdominal organs receive sympathetic innervation from postganglionic fibers originating from sympathetic (cervical, thoracic, and splanchnic) ganglia.
1.1.2 The Parasympathetic ANS
A scheme of the parasympathetic ANS is illustrated in Fig. 1.1. The parasympathetic ANS division has craniosacral “outflow,” i.e., the preganglionic efferent neurons leave the central nervous system through cranial nerves (III, VII, IX, X) or together with the anterior roots of sacral spinal nerves (mainly S2–S3, but also S1 and S4). Cell bodies of cranial parasympathetic preganglionic neurons are located in some nuclei of the brainstem, whereas those of sacral neurons are located at the base of the intermediolateral portion of the anterior horns of the gray substance of the respective spinal segments.
Parasympathetic postganglionic neurons have cell bodies located in parasympathetic ganglia, which are always found peripherally, next to, or also within, the target organs, and are always reached by preganglionic parasympathetic fibers through somatic nerves.
It is worth noting that 75 % of all parasympathetic nerve fibers are situated in the vagus nerve (X cranial nerve). Vagal fibers mostly originate from the dorsal motor nucleus and, in part, from the ambiguous nucleus. The vagus nerve sorts out of the central nervous system through the jugular foramen at each side and travels down until the abdomen, giving branches to thoracic and upper abdominal organs.
1.1.3 Afferent ANS Fibers
As for the somatic sensitive system, signals from visceral organs are transmitted by primary sensory neurons. The afferent fibers originated from visceral organs travel in sympathetic and parasympathetic nerves and transmit information to the central nervous system about activities of the organ or the occurrence of tissue injury, the latter through nociceptive fibers. These signals also generate autonomic reflexes which allow regulation of organ functions. Some of these signals (e.g., pain signals) can be transmitted to cortical centers and become conscious.
Nociceptive fibers are mainly associated with sympathetic nerves. Their cell bodies are located in spinal ganglia, achieved through the SNC and rami communicantes, and they end in the posterior roots of the spinal cord, where they synapse on “second-order” nociceptive neurons. Nociceptive visceral fibers are much less numerous than nociceptive somatic fibers and usually end at more levels in the spinal cord, thus generating less specific and localized pain sensation.
Afferent primary sensory fibers associated with parasympathetic nerves are more involved in regulatory reflexes of system/organ activities. Again, they are mainly associated with the vagus nerve, but several visceral cranial afferent fibers travel with the glossopharyngeal or facial nerves and pelvic fibers with pelvic nerves. The cell bodies of these afferent fibers are located in ganglia associated with these parasympathetic nerves. Primary sensory neurons then project onto “second-order” visceral sensory neurons located in the medulla oblongata, in the nucleus of the solitary tract, and other nuclei.
1.1.4 The Enteric ANS
It is worth noting that enteric ganglia diffused inside the wall of the digestive tube collectively contain as many neurons as the entire spinal cord, including local sensory neurons, motor neurons, and interneurons, and is able to act as a largely autonomous part of the ANS. For this reason, the enteric ANS is often considered as a third, independent part of the ANS.
1.2 Function of the ANS
A description of the functions of the ANS is beyond the scope of this chapter, and therefore some general concepts only are discussed here.
The sympathetic and parasympathetic systems are generally thought to act in opposition to each other. However, their effects in most organs should be better considered as complementary in nature rather than antagonistic, with most visceral functions resulting from the balance of the influence of the two ANS branches (Guyton and Hall 2006). Furthermore, some viscera (e.g., visceral vessels, liver, spleen) only receive sympathetic innervations, and in other cases sympathetic and parasympathetic nerves have synergic effects (e.g., increase of salivary gland secretion).
Overall, however, the sympathetic ANS is typically activated in conditions of stress and is responsible for the so-called “fight-or-flight” response, which is characterized by enhanced heart rate and contractility, dilation of coronary vessels, bronchiole dilation and increased alveolar oxygen exchange, increased blood flow to skeletal muscles, and reduction of blood flow to the gastrointestinal tract and skin.
The parasympathetic ANS, instead, predominates in basal conditions, defining the so-called “rest and digest” status, which is characterized by dilation of blood vessels and accelerated peristalsis of the gastrointestinal tract, together with reduction of cardiac and respiratory activities.
The peripheral effects of sympathetic and parasympathetic systems are mediated by the release from nerve endings of specific chemical neurotransmitters, which act on specific receptors on cell membranes of target organs.
The neuromediator released by peripheral sympathetic fibers and therefore responsible for their effects is norepinephrine, while acetylcholine is the neuromediator released by parasympathetic nerve fibers (Fig. 1.3). Together with these primary neuromediator, both sympathetic and parasympathetic nerve fibers can variably release other substances, mainly neuropeptides, which can contribute to the final effects of nerve stimulation. Examples of these substances include neuropeptide Y, galanin, and dynorphin in noradrenergic fibers, and vasoactive intestinal peptide, calcitonin gene-related peptide, and substance P in cholinergic fibers (Jänig 2006). The exact role of most of these co-released substances, however, remains to be defined.

Fig. 1.3
Chemical structure of the sympathetic (norepinephrine) and parasympathetic (acetylcholine) neurotransmitters
It is also worth noting that postganglionic sympathetic fibers for sweat glands and for skeletal muscle vessels release acetylcholine and that acetylcholine is also the neurotrasnmitter of both sympathetic and parasympathetic signals by preganglionic neurons in the respective ganglia.
Norepinephrine exerts its effects through binding and activation of adrenergic receptors on target cells. Two main classes of adrenergic receptors have been described, the α- and β-receptors (Ahlquist 1948). The α-adrenergic receptors present two main types α1- and α2-receptors. Similarly, two main types of β-adrenergic receptors have been found, β1- and β2-receptors, although a third type (β3) has recently been found to also play some relevant physiological role (Bylund et al. 1994).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


