Chapter 62 The Aorta
Aneurysmal Disease
Aneurysms, defined as an increase in size of more than 50% of the normal arterial diameter, may occur anywhere in the aorta, from the aortic root to the bifurcation. Although most nonruptured aneurysms are asymptomatic, a number of risk factors for the development, expansion, and rupture of an abdominal aortic aneurysms (AAA) have been identified (Table 62-1). Risk factors for developing an AAA include age, male gender, family history, tobacco use, hypertension, hyperlipidemia, and height. Aneurysm development is also associated with the presence of connective tissue disorders, such as Marfan syndrome, and of concurrent aneurysmal or atherosclerotic disease.1–11 Aneurysmal enlargement of the aorta is associated with factors that result in weakening of the arterial wall and increased local hemodynamic forces. These may include heritable conditions such as Marfan syndrome, familial thoracic aortic aneurysm and dissection (TAAD), and vascular-type Ehlers-Danlos syndrome, as well as less well-defined entities that contribute to the significantly elevated incidence in patients with a family history of aneurysm. Factors that contribute to the degradation of collagen and elastin are also associated with aneurysmal disease; research in this area has focused on the role of matrix metalloproteinases (MMPs) and other mediators of tissue enzyme function. The immune response has also been implicated in the pathophysiology of aneurysm formation.12
Table 62-1 Risk Factors for Aneurysm Development, Expansion, and Rupture
| SYMPTOM | RISK FACTORS |
|---|---|
| AAA development | Tobacco use |
| Hypercholesterolemia | |
| Hypertension | |
| Male gender | |
| Family history (male predominance) | |
| AAA expansion | Advanced age |
| Severe cardiac disease | |
| Previous stroke | |
| Tobacco use | |
| Cardiac or renal transplant | |
| AAA rupture | Female gender |
| ↓ FEV1 | |
| Larger initial AA diameter | |
| Higher mean blood pressure | |
| Current tobacco use (length of time smoking >> amount) | |
| Cardiac or renal transplantation | |
| Critical wall stress–wall strength relationship |
Adapted from Chaikof EL, Brewster DC, Dalman RL, et al: The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guidelines. J Vasc Surg 50:S2–S49, 2009.
Diagnosis
An abdominal aneurysm may be detected on physical examination as a palpable pulsatile mass, most commonly supraumbilical and in the midline. The location may, however, be variable, because aortic tortuosity may render the palpable mass lateral and/or infraumbilical. The sensitivity of physical examination is, as one might expect, dependent on aneurysm size and patient habitus.1
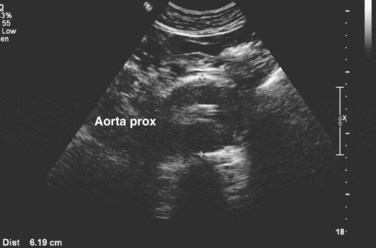
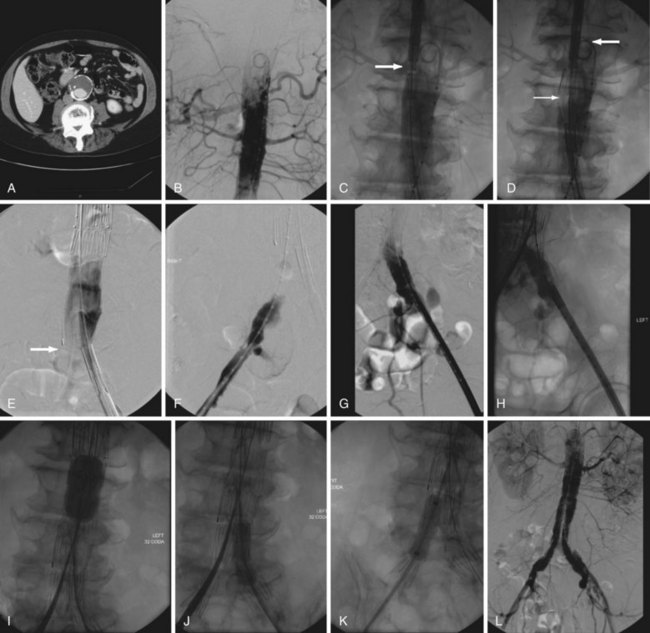
The detection and characterization of aneurysms may be greatly aided by modern imaging techniques. Ultrasound examination has been demonstrated to afford excellent sensitivity and specificity (Fig. 62-1).1 Ultrasound may be limited by patient habitus or bowel gas, but because it avoids the complications associated with invasive testing, radiation, and contrast media, is an excellent choice for screening. It must also be noted that ultrasound is not an ideal method for detecting rupture, because ultrasound cannot image all portions of the aortic wall and the nonfasting status of emergently examined patients may further preclude ideal image acquisition. It has been estimated that ultrasound may fail to detect up to 50% of aneurysm ruptures.
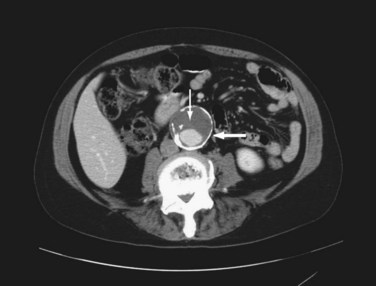
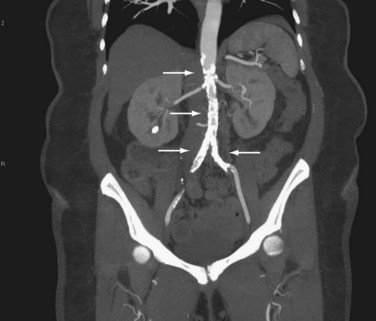
Computed tomography (CT) provides excellent imaging of AAA, with greater reproducibility of diameter measurements than ultrasound.1 CT, particularly with the adjunctive use of iodinated contrast to carry out CT angiography (CTA), provides a wealth of anatomic information, detects vessel calcification, thrombus, and concurrent arterial occlusive disease, and permits multiplanar and three-dimensional reconstruction and analysis for operative planning (Fig. 62-2). Drawbacks include substantial radiation exposure, particularly in the setting of serial examinations, and the use of iodinated contrast media in a population with a high incidence of comorbid kidney disease.
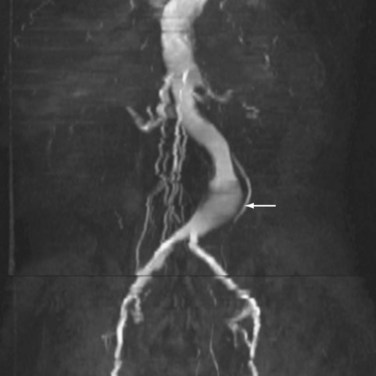
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are, like CT, sensitive for the detection of AAA (Fig. 62-3). Unlike CT, MRI does not demonstrate aortic wall calcification, which may be important in operative planning. Although the study does not require the use of iodinated contrast, MRA uses gadolinium, which has been associated with the development of nephrogenic systemic fibrosis in patients with a low glomerular filtration rate (GFR). The ability to acquire dynamic images throughout the cardiac cycle may ultimately prove clinically useful.13
Risk of Rupture
Guidelines for surveillance and treatment of AAA are based on data regarding risk factors for rupture. Published risk factors for rupture include chronic obstructive pulmonary disease (COPD), current tobacco use, larger initial AAA diameter, female gender, cardiac or renal transplantation, and certain patterns of wall stress.1,14–23 The most widely adopted surrogate for rupture risk is maximal cross-sectional aneurysm diameter (Table 62-2).
Table 62-2 Estimated Annual Rupture Risk
| AAA DIAMETER (cm) | RUPTURE RISK (%/yr) |
|---|---|
| <4 | 0 |
| 4-5 | 0.5-5 |
| 5-6 | 3-15 |
| 6-7 | 10-20 |
| 7-8 | 20-40 |
| >8 | 30-50 |
Adapted from Brewster DC, Cronenwett JL, Hallett JW Jr, et al: Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 37:1106–1117, 2003.
In addition, despite a relative paucity of natural history data regarding growth rate and rupture, most clinicians incorporate into practice the concept of rate of enlargement as a risk factor for rupture. In this case, a rate of growth of more than 5 to 7 mm/6 months or more than 1 cm/year has been widely adopted as an indication for repair, independent of aneurysm size. It is important to note that size is an imperfect predictor of rupture risk, because autopsy studies have discovered evidence of rupture in up to 12% of aneurysms less than 5 cm in diameter.24 There are a number of investigational models that have attempted to quantify rupture risk based on calculations of wall stress or the combination of multiple factors thought to contribute to increased wall stress and/or decreased strength.
Screening and Surveillance Recommendations
Screening recommendations for AAA are based on the sensitivity and specificity of ultrasound screening, detection yield of screening based on various risk factor selection criteria, and cost. A major recent compilation of evidence-based recommendations for screening and surveillance of abdominal aortic aneurysms has been provided by practice guidelines developed by the Clinical Practice Council of the Society for Vascular Surgery (SVS).1 The SVS committee charged with reviewing available data regarding screening made a strong recommendation for one-time screening of all men aged 65 years or older or men 55 or older with a family history of AAA. Screening of women is also strongly recommended for those aged 65 years or older with a family history of AAA or a personal smoking history. The evidence basis of these recommendations was deemed to be strong in the former case and moderate in the latter. The U.S. Preventive Services Task Force has issued a more limited recommendation for one-time screening of men between 65 and 75 years of age who have a personal smoking history.25 It is important to note that payer policies regarding reimbursement may not track of these recommendations. Medicare, for example, as a result of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAVE) Act, covers screening for select populations (men with a personal smoking history and men or women with a family history of AAA), but only as a part of the initial welcome to Medicare physical examination.
Once an aneurysm has been detected, the SVS Clinical Practice Council recommends further screening intervals as follows, based on aneurysm size (maximum external aortic diameter) and associated risk of rupture1:
Treatment
Medical Therapy
Once an aneurysm has been diagnosed, a number of measures may be taken to optimize the patient’s medical regimen and potentially minimize the rate of aneurysm expansion and rate of rupture. As noted, current tobacco use has been associated with an increased rate of aneurysm expansion. Smoking cessation may also yield benefits with regard to perioperative morbidity and mortality in the event that the aneurysm ultimately requires repair. Control of blood pressure and rate of increase of left ventricular pressure (dP/dT) has been proposed as being important in minimizing wall stress that may contribute to aneurysm expansion or rupture. However, studies of beta blockade with propranolol to accomplish both these goals failed to show an effect on aneurysm expansion. More recently, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), statins, and antibiotics (e.g., doxycycline) have been associated with a decreased rate of expansion.26
Surgical Treatment
Surgical treatment is generally recommended for aneurysms larger than 5.5 cm in maximal diameter, those demonstrating more than a 5-mm growth in 6 months or more than 1 cm in 1 year, and aneurysms with a saccular rather than the typical fusiform anatomy. However, significant gender differences in the natural history of AAA have emerged, with research suggesting that although women develop aneurysms somewhat less frequently than men, aneurysm prevalence increases sharply with age in women. In addition, there is evidence that aneurysms in women exhibit more rapid growth and rupture at smaller sizes (average diameter of 5 cm in female versus 6 cm in male patients).27
Preoperative Evaluation
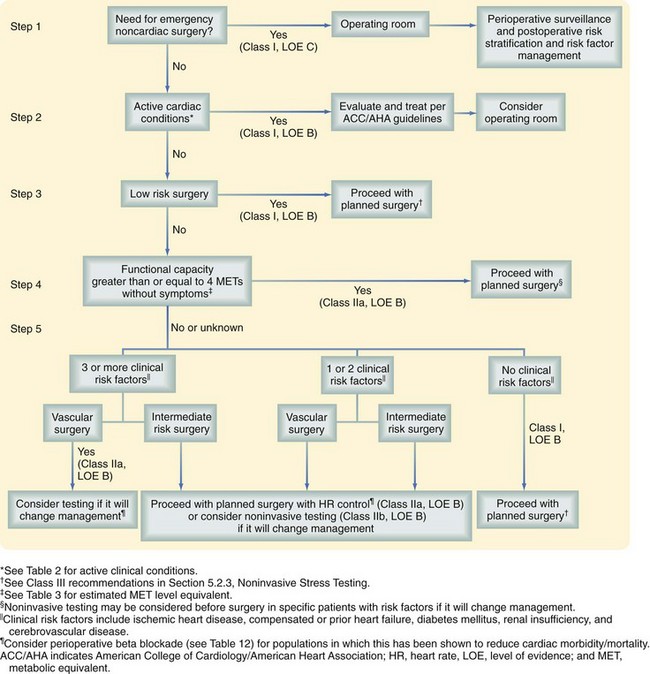
The preoperative evaluation of patients with AAA comprises operative planning as well as the identification and management of important medical comorbidities, such as coronary artery disease (CAD), renal insufficiency, peripheral arterial occlusive disease, diabetes, and obstructive lung disease. Because CAD is the primary cause of mortality following open or endovascular repair of AAA, much attention has been focused on the preoperative evaluation and management of comorbid CAD. The guiding principles in this evaluation have traditionally been the identification of information that will alter management and the institution of therapy that will improve cardiac-related mortality. In 2007, the American College of Cardiology/American Heart Association (ACC/AHA) published guidelines regarding the preoperative cardiac evaluation of patients undergoing noncardiac vascular surgery.28 These guidelines stratify patients according to the presence or absence of symptomatic cardiac disease, presence of significant clinical risk factors (e.g., mild angina, prior myocardial infarction [MI], compensated congestive heart failure [CHF], diabetes mellitus, renal insufficiency), and the level, quantified as metabolic equivalent (MET), of the patient’s functional capacity. All patients should be assessed with a resting electrocardiogram (ECG). Echocardiography may be used to evaluate the cardiac function of those with a history of heart failure or current dyspnea. The decision to proceed with noninvasive testing in patients without symptoms of active cardiac disease should be based on patient functional capacity and the presence of three or more significant additional risk factors. Coronary angiography should be considered for patients with evidence of active cardiac disease based on screening questions or evidence of ischemia on noninvasive stress testing. Adjunctive medical therapy may also serve to reduce the risk of perioperative cardiac events. Perioperative beta blockade, statin use, and aspirin use are widely accepted; there is also evidence to support the use of other antihypertensives during this period (Fig. 62-4).1
Renal insufficiency related to renovascular or medical renal disease is a well-established risk factor for morbidity and mortality following AAA repair. Coexisting renal artery occlusive disease may be present in 20% to 38% of patients with AAA.29 Also, open and endovascular repair of AAA may result in further deterioration in the renal function of patients with preexisting renal disease. Concurrent repair of clinically significant renal occlusive disease is appropriate at the time of open or endovascular aneurysm repair. Various strategies for intraoperative renal protection have been proposed. Current recommendations include adequate hydration, perioperative discontinuation of ACEIs and ARBs, and avoidance of hypotension. There is mixed evidence regarding the benefits of antioxidants (e.g., mannitol, ascorbic acid, vitamin E, N-acetylcysteine, allopurinol) and there are some data supporting the beneficial effects of infused fenoldopam.30,31 When suprarenal clamp placement is necessary, we endorse the use of cold saline perfusion of the kidneys, preclamp administration of furosemide (Lasix) and mannitol, and selective use of fenoldopam. An additional consideration, particularly in patients with preexisting renal dysfunction, is contrast-induced nephropathy (CIN) associated with the administration of iodinated contrast agents for CT imaging or angiography. Current data support IV hydration with sodium bicarbonate or normal saline and possibly the use of antioxidants, such as ascorbic acid or N-acetylcysteine. When EVAR is contemplated, carbon dioxide may be used as an imaging agent to alleviate or minimize the need for iodinated agents, because the rate of CIN is related to the amount of agent administered, age, and prior renal function.
Data are mixed with regard to the impact of pulmonary disease, particularly COPD, on mortality following AAA repair. However, there is evidence that optimal management of comorbid COPD may improve morbidity and mortality.32 We support obtaining a preoperative pulmonary function assessment, including arterial blood gases, to assess risk and guide management in the perioperative period. Patients with poor pulmonary function must be made aware of the increased risk that they will require prolonged ventilatory support postoperatively and the attendant possibility that tracheostomy will be required during this period. Smoking cessation prior to surgery may be beneficial; this can be aided by counseling and a variety of pharmacologic therapies. Although several studies have suggested that initiating smoking cessation less than 2 weeks before surgery may actually be associated with worse outcomes, a recent meta-analysis has suggested that smoking cessation at any period of time within 8 weeks of surgery is not associated with a higher rate of overall complications or pulmonary complications postoperatively.33
Careful evaluation of preoperative imaging is crucial when planning repair. Anatomic variations such as a retroaortic renal vein, variant inferior vena cava, or horseshoe kidney may significantly affect the selection of surgical approach and, if not appreciated preoperatively, can lead to disastrous complications. CT affords the additional advantage of demonstrating vascular calcification, thus permitting the surgeon to assess the feasibility of clamping the aorta and iliac arteries at various levels (Fig. 62-5). Occlusion balloons may be substituted for arterial clamp placement, most frequently at the iliac arteries, should severe calcification render clamp placement untenable. Finally, the size and patency of branch vessels, such as the inferior mesenteric, accessory renal, iliac, and lumbar arteries, can be assessed and may further contribute to preoperative planning.
Technique of Open Surgical Repair of Abdominal Aortic Aneurysms
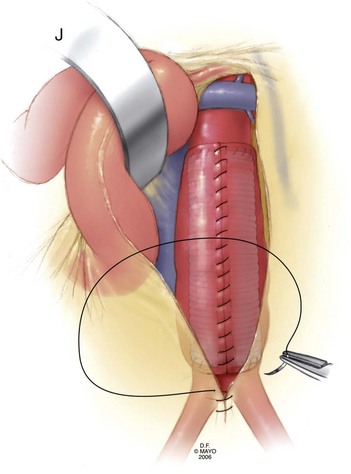
The infrarenal transperitoneal repair begins with the administration of perioperative antibiotic, typically a first-generation cephalosporin, and scrupulous skin preparation from the nipples to the thighs. If treating a ruptured aneurysm, skin preparation and draping are accomplished prior to the induction of general anesthesia to permit rapid exposure and control if induction incites hemodynamic collapse. The patient is draped and a generous midline laparotomy incision is made from the xiphoid to just above the pubis. Extension of this incision along the xiphoid may facilitate supraceliac exposure, if necessary. If repair is elective and preoperative imaging has demonstrated iliac disease necessitating extension of a bifurcated graft to the femoral artery on one or both sides, the femoral artery dissection should be accomplished prior to laparotomy (Fig. 62-6).
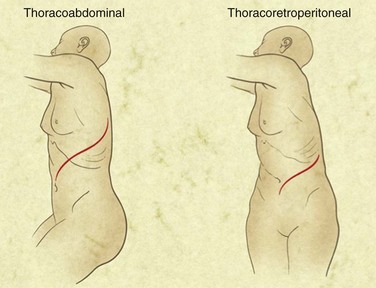
The retroperitoneal approach is thought by some to reduce physiologic stress on the patient and to result in fewer postoperative pulmonary complications, as well as a reduction in postoperative ileus.34 Both approaches are associated with a significant rate of wound healing complications. Midline incisions for AAA repair were complicated by radiographically apparent abdominal wall defects in approximately 20% of cases in a recent series, although clinically significant hernias are less frequent. Persistent postoperative pain, flank wall laxity, and hernia have been described as complicating retroperitoneal repair and some investigators have reported more frequent occurrence of these complications using the retroperitoneal technique. With regard to operative exposure, the retroperitoneal approach does afford greater access to the visceral segment of the abdominal aorta and may be aided, if required, by thoracic extension of the incision and exposure with or without division of the diaphragm.
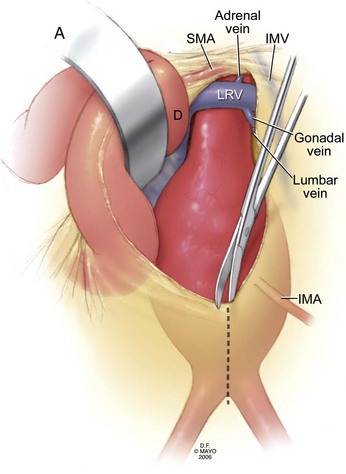
A retroperitoneal aortic exposure may be accomplished with the patient in a modified right lateral decubitus position, with the thorax rotated but the hips relatively flat to permit access to both groins (Fig. 62-7). A curvilinear incision is made from the costal margin to below the umbilicus, depending on the extent of exposure required and patient habitus. The retroperitoneal plane may be entered at the lateral border of the rectus sheath. The rectus abdominus may be reflected medially or laterally. Some surgeons prefer lateral reflection, because this may result in less difficulty with postoperative body wall laxity. Care is taken to avoid entering the peritoneum. Much of the initial portion of this dissection may be carried out bluntly, with the aid of a tonsil sponge on a ring or Kelly forceps. The abdominal contents, enveloped in peritoneum, may be swept medially. The ureter will be visualized and swept medially. The left kidney may be elevated or left in situ, although we generally prefer to medialize the kidney, which also serves to mobilize the left renal vein. The gonadal tributary, however, must generally be identified, ligated, and divided. Proximally, the spleen is carefully mobilized within its peritoneal covering to expose the underside of the diaphragm. The fibers of the left crus of the diaphragm, when divided, expose the supraceliac and visceral portions of the aorta. The left renal artery should be readily accessible and the celiac and superior mesenteric arteries may be mobilized by careful dissection. The right renal artery is frequently difficult to isolate prior to aortotomy. Distally, the iliacs are carefully exposed in the avascular plane by gently mobilizing overlying structures, including the ureters. Again, the full exposure of the right iliac is typically more difficult by this approach, depending on patient habitus. The extensive exposure of the supraceliac and visceral portions of the aorta permit full access and nuanced decision making regarding clamp placement, which may be suprarenal, supramesenteric, or supraceliac. Visceral and renal vessels may be controlled by clamp placement, vessel loops or, after aortotomy, use of occlusion balloons, with great care taken in handling to avoid dissection or embolization. Occlusive disease or aneurysmal involvement of renal or visceral vessels may be readily addressed by this approach. According to patient indications and surgeon preference, cardiopulmonary bypass may be used as an adjunct; this provides the ability to perfuse the renal and visceral vessels actively if a complex or prolonged reconstruction is anticipated.
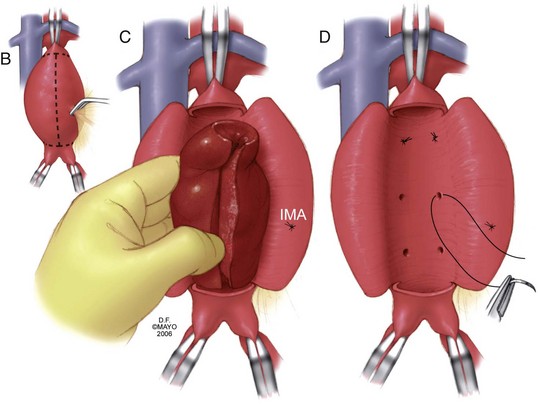
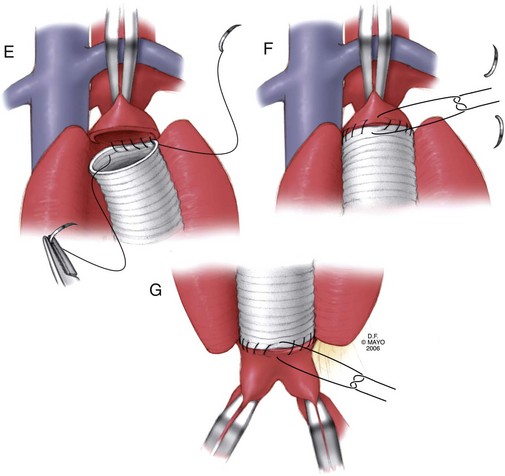
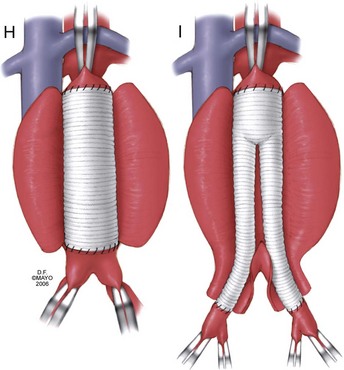
Once adequate exposure has been achieved, proximal and distal clamps may be placed. As in the transperitoneal approach, repair is typically accomplished by endoaneurysmorrhaphy using end-to-end proximal and distal anastomoses to replace the diseased portion of the aorta as an interposition. Once again, the aneurysm thrombus is removed at the time of aortotomy and lumbar arteries are ligated within the sac. The same principles of backbleeding and flushing of the graft prior to completion of the distal anastomosis apply. This approach also permits a variety of approaches to reconstruction of the juxtarenal, pararenal, and paravisceral aorta. Branch vessels may be incorporated together by careful beveling of the graft, reimplanted individually as Carrel patches, or reconstructed using short bypass grafts. When treating thoracoabdominal aneurysms, the incision may be extended into the chest at the appropriate rib space and the diaphragm circumferentially divided to afford enough exposure to extend the repair to almost any level of the descending aorta. The rib may be circumferentially dissected and divided posteriorly to improve thoracic exposure further, as needed. When hemostasis is achieved, the sac may, again, be closed over the graft, although the retroperitoneally placed graft is not as vulnerable to erosion and aortoduodenal fistula as that placed transperitoneally (Fig. 62-8).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree