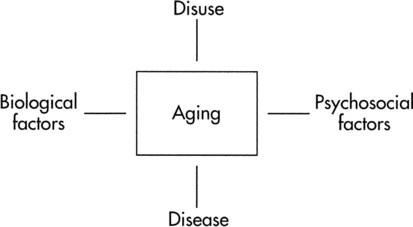
An additional issue is a model of aging that might sort out some of the determinants of aging (Figure 38-1). The model identifies biological factors, disuse, pathology, and psychosocial concerns as determinants of aging. Biological factors address genetics, sex, cellular mechanisms, and metabolic and physiological responses that influence aging. Disuse is implicated in the more sedentary lifestyle led by many older adults, which results in loss of exercise capacity.1 With exercise training, it should be possible to reverse or attenuate capacities that decline as a result of disuse. This chapter focuses on the biological and disuse characteristics of aging. The impact of various pathological conditions is discussed in other chapters. Psychosocial issues related to exercise and aging are not addressed. Considerable disagreement exists regarding the mechanisms that contribute to the decline in Age-related changes in cardiac structure occur in the form of left ventricular wall thickness13,15 and are attributed to an increase in size of cardiac myocytes16 and increased collagen.13 Additional cardiac structural changes include increases in vascular intimal thickness, vascular stiffness, and left arterial dimension.13,17 The diastolic properties (cardiac filling) of the heart alter with age. Diastole requires relaxation of the myocardial fibers, sufficient venous return to rapidly fill the heart, and timing of the atrial contraction to contribute to the end-diastolic volume. Relaxation may be hampered by increased ventricular stiffness, although there is limited evidence of this in humans.14 The period of the isovolumetric myocardial relaxation (the time between aortic valve closing and mitral valve opening) is prolonged with aging.15 Likewise, the peak rate of left ventricular filling during early diastole is progressively reduced so that between the ages of 20 and 80 years, the average rate can be reduced up to 50%.13 Despite the changes in early diastole, the resting left ventricular end-diastolic volume remains the same because of an enhanced left atrial contribution to ventricular filling.13 This is accompanied by an enlarged left atrium and an audible fourth heart sound in most older adults.13,18 Considerable disagreement exists regarding what happens to diastolic function during exercise. End-diastolic volume index (end-diastolic volume normalized for body surface) increases similarly in both young and older men during submaximal exercise; however, only older men remain at these elevated levels during exhaustive exercise.13 Filling pressures during exercise in men increase with age.19 In addition, peak left ventricular diastolic filling rate during submaximal and maximal exercise decreases with aging.20,21 Decreased filling rate is associated with increased ventricular stiffness and prolonged relaxation time.22 Resting measures of systolic and cardiac pump function do not change with aging. The resting end-systolic volume and stroke volume do not change with age. Likewise, ejection fraction at rest (end-diastolic volume − [end-systolic volume/end-diastolic volume]) is similar in healthy older and younger individuals.13 Unlike resting systolic function, the pumping function of the heart changes considerably in response to exercise. Myocardial contractility as measured by the ratio of end-systolic volume to systolic arterial pressure declines during exercise as people age.14 The end-systolic volume index increases, whereas the ejection fraction decreases during exercise.13 Reduced contractile performance is related to decreased response to beta-adrenergic stimulation, changes in the myocardium, increased systolic blood pressure, and ventricular wall abnormalities.8 For older people who remain active, the rate of decline in The mechanisms underlying improvements in A recent study followed a small group of competitive distance-running men for 10 years with measures of the cardiovascular, pulmonary, and metabolic systems.35 The 5-kilometer race time slowed over the 10 years, along with decreased training mileage, intensity, and frequency. Consistent with this, the peak exercise heart rate and oxygen consumption declined. Another intriguing study indicates that telomere length, a measure of the ability of cells to replicate, is preserved in healthy, older adults who engage in vigorous aerobic exercise.36 The preservation of telomere length is positively related to maximum oxygen consumption, suggesting that exercise reduces cell senescence with aging. Further studies examining the outcomes of prolonged, sustained endurance training in older men and women are needed to characterize the adaptations that occur in women. The diastolic changes that occur with aging can be reversed with exercise training.20 Levy and colleagues intensively endurance trained 13 rigorously screened older men aged 60 to 82 years (mean age 68 years) and 11 younger men in their 20s, for 6 months. The training produced increased resting, submaximal, and peak filling rates for the older group comparable to the changes observed in the younger group.20 End-diastolic peak volume at rest and exercise also increase after lengthy endurance training.30 Thus training may reduce the age-associated diastolic changes. In contrast, endurance athletes who were followed for 10 years after age 65 showed deterioration in diastolic left ventricular function indices consistent with decreased cardiovascular diastolic function.35 Another study demonstrated that 1 year of vigorous exercise training in sedentary seniors did not improve cardiac stiffening, but improved left ventricular mass and reduced arterial elastance.37 The mechanisms of these responses are uncertain in humans. Studies in rats have shown that exercise training increases calcium uptake in cardiac sarcoplasmic reticulum, decreases relaxation time, reduces the decline of left ventricular pressure, enhances fatty acid oxidation, and increases cytochrome c oxidase levels.20,38,39 All these changes have been associated with reduced diastolic function with aging. Exercise training can also improve systolic performance in older men as reflected by the increase in exercise stroke volume. Increased peak stroke volume occurs with an increased exercise ejection fraction, a decrease in end-systolic volume, and greater left ventricular wall mass.3,30,31 These changes apparently do not occur in older, estrogen-deficient women.33 Table 38-1 summarizes these changes. Table 38-1
The Aging Patient
Cardiac Changes with Aging
Aerobic Capacity
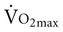 declines with age2,3 at a rate of 0.40 to 0.50 mL/kg/min per year for men and between 0.20 to 0.35 mL/kg/min per year for women (Figure 38-2).4 The reduction is approximately 10% per decade. The decline is faster and greater in men than women; however, men have a larger aerobic capacity overall than women.5,6
declines with age2,3 at a rate of 0.40 to 0.50 mL/kg/min per year for men and between 0.20 to 0.35 mL/kg/min per year for women (Figure 38-2).4 The reduction is approximately 10% per decade. The decline is faster and greater in men than women; however, men have a larger aerobic capacity overall than women.5,6 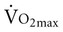 is related to body size, which is generally smaller in women than men.
is related to body size, which is generally smaller in women than men. 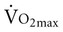 is associated with peripheral vascular reserve in older men but not in women.7 Increases in body weight along with aging result in reduced
is associated with peripheral vascular reserve in older men but not in women.7 Increases in body weight along with aging result in reduced 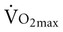 , even if aerobic capacity remains the same, because relative oxygen consumption is related to body weight. Reduced physical activity with aging also contributes to a loss of
, even if aerobic capacity remains the same, because relative oxygen consumption is related to body weight. Reduced physical activity with aging also contributes to a loss of 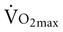 .
.
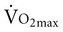 with age. Both cardiac and peripheral changes contribute to this loss. Maximum cardiac output declines with age similarly in men and women.7 A reduction in maximum cardiac output may account for 50% to 100% of the total reduction in
with age. Both cardiac and peripheral changes contribute to this loss. Maximum cardiac output declines with age similarly in men and women.7 A reduction in maximum cardiac output may account for 50% to 100% of the total reduction in 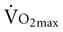 .8–10 McGuire and colleagues (2001)2, however, suggested that reductions in peripheral oxygen extraction were the dominant mechanism responsible for reduced
.8–10 McGuire and colleagues (2001)2, however, suggested that reductions in peripheral oxygen extraction were the dominant mechanism responsible for reduced 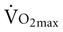 with age. A major component to the decline in maximum cardiac output is decreased maximum heart rate.3,9,11 The decline in heart rate is linearly related to age and occurs in both sedentary and active people.5,12 Discrepancies in the age-related response of maximum stroke volume also exist; studies have reported reduced, preserved, and increased maximum stroke volume with age.2,3,9,13 The decline in
with age. A major component to the decline in maximum cardiac output is decreased maximum heart rate.3,9,11 The decline in heart rate is linearly related to age and occurs in both sedentary and active people.5,12 Discrepancies in the age-related response of maximum stroke volume also exist; studies have reported reduced, preserved, and increased maximum stroke volume with age.2,3,9,13 The decline in 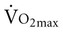 associated with aging is most likely attributable to decreased maximum heart rate, stroke volume, and arteriovenous oxygen content difference, although each component’s contribution varies.8,14
associated with aging is most likely attributable to decreased maximum heart rate, stroke volume, and arteriovenous oxygen content difference, although each component’s contribution varies.8,14
Cardiac Mechanics
Exercise Training
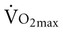 is reduced to 5% per decade, compared with an anticipated decline of 10% per decade in sedentary adults.11 A meta-analysis of 29 studies on endurance training, which included 1030 men and 466 women between the ages of 61 and 78 years, concluded that endurance training increases functional capacity in young older adults.23 Less improvement was seen with increasing age, a shorter length of training, low
is reduced to 5% per decade, compared with an anticipated decline of 10% per decade in sedentary adults.11 A meta-analysis of 29 studies on endurance training, which included 1030 men and 466 women between the ages of 61 and 78 years, concluded that endurance training increases functional capacity in young older adults.23 Less improvement was seen with increasing age, a shorter length of training, low 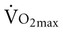 before training, and short duration of exercise sessions. The analysis suggests that a healthy 68-year-old individual who exercises for 30 minutes three times per week for 4 to 6 months can improve
before training, and short duration of exercise sessions. The analysis suggests that a healthy 68-year-old individual who exercises for 30 minutes three times per week for 4 to 6 months can improve 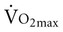 by 14%.23 Similar improvements occur in both men and women.24,25
by 14%.23 Similar improvements occur in both men and women.24,25
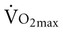 in older adults who engage in endurance training are not clear. One consistent finding, however, is greater extraction of oxygen in the exercising skeletal muscle, which produces a wider arteriovenous oxygen content difference in both older men and women.9,22,26 This implies that adaptations in the peripheral skeletal muscles account for some of the increase in
in older adults who engage in endurance training are not clear. One consistent finding, however, is greater extraction of oxygen in the exercising skeletal muscle, which produces a wider arteriovenous oxygen content difference in both older men and women.9,22,26 This implies that adaptations in the peripheral skeletal muscles account for some of the increase in 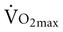 in older adults. The impact of exercise training on maximum cardiac output is uncertain.22 Maximum cardiac output can either remain the same or increase after exercise training, depending on the effect of training on maximum stroke volume and maximum heart rate. Maximum heart rate remains the same in older men regardless of activity level, suggesting that the decline in maximal heart rate depends on factors other than exercise and physical fitness.27 A decrease in response to circulating catecholamines is most often cited as the reason for changes in maximum heart rate with aging.28 Relatively intense endurance training for a year or more can increase peak stroke volume in men.29–31 Adaptations to exercise training in older women have been attributed predominantly to peripheral changes in arteriovenous oxygen content difference rather than central changes in cardiac function.32,33 This apparently occurs despite intensive endurance training over a year-long period. In contrast, a relatively recent study that compared men and women who were masters athletes with healthy sedentary older adults, healthy young persons, and sedentary controls reported that stroke volume for any given filling pressure was greater for masters athletes compared with age-matched sedentary older adults.34
in older adults. The impact of exercise training on maximum cardiac output is uncertain.22 Maximum cardiac output can either remain the same or increase after exercise training, depending on the effect of training on maximum stroke volume and maximum heart rate. Maximum heart rate remains the same in older men regardless of activity level, suggesting that the decline in maximal heart rate depends on factors other than exercise and physical fitness.27 A decrease in response to circulating catecholamines is most often cited as the reason for changes in maximum heart rate with aging.28 Relatively intense endurance training for a year or more can increase peak stroke volume in men.29–31 Adaptations to exercise training in older women have been attributed predominantly to peripheral changes in arteriovenous oxygen content difference rather than central changes in cardiac function.32,33 This apparently occurs despite intensive endurance training over a year-long period. In contrast, a relatively recent study that compared men and women who were masters athletes with healthy sedentary older adults, healthy young persons, and sedentary controls reported that stroke volume for any given filling pressure was greater for masters athletes compared with age-matched sedentary older adults.34
Because of Aging
After Exercise Training
MAXIMUM EXERCISE
Oxygen consumption
↓
↑
Heart rate
↓
↔
Stroke volume
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

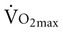 ), or the maximum amount of oxygen used during exercise.
), or the maximum amount of oxygen used during exercise. 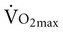 is directly related to cardiac output (the amount of blood pumped by the heart) and the arteriovenous oxygen content difference (the amount of oxygen extracted in the periphery). Cardiac output is the product of heart rate and stroke volume. Aerobic capacity reflects the central cardiac function and the efficiency of the peripheral tissues to extract and use oxygen.
is directly related to cardiac output (the amount of blood pumped by the heart) and the arteriovenous oxygen content difference (the amount of oxygen extracted in the periphery). Cardiac output is the product of heart rate and stroke volume. Aerobic capacity reflects the central cardiac function and the efficiency of the peripheral tissues to extract and use oxygen.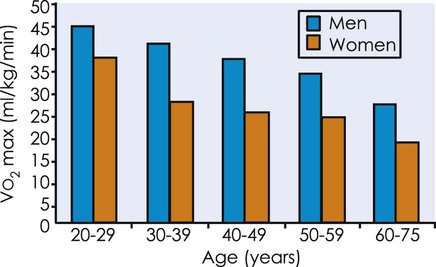
 from 20 to 75 years of age in both men and women.
from 20 to 75 years of age in both men and women.