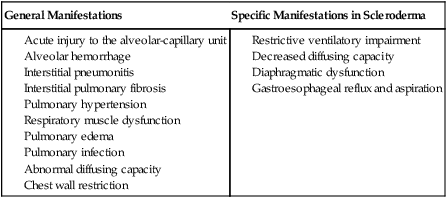
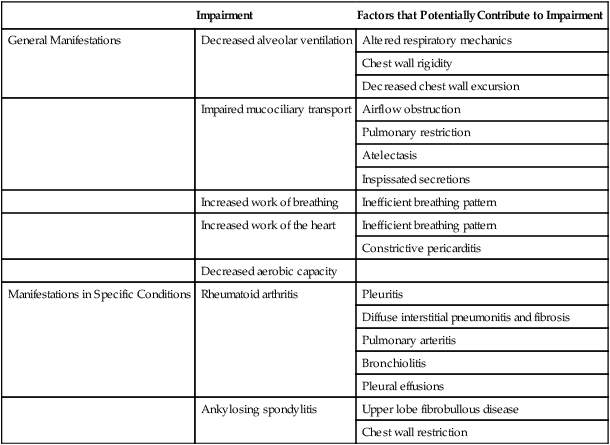
To maximize the effectiveness of their treatment prescriptions, physical therapists need to be able to predict the impact of systemic conditions on oxygen transport in a given patient.1 Complications due to systemic conditions are increasingly prevalent. This may reflect both the aging of the population and the improved survival rate and prognosis of patients with multisystem comorbidity. In addition, it is becoming more common for physical therapists to treat patients without referral, in which case no referring practitioner is alerting the physical therapist about the presence and significance of underlying systemic conditions. In their role as clinical exercise physiologists, physical therapists must be able to identify all factors that compromise or threaten oxygen transport so that treatment interventions can be prescribed effectively and with minimal risk. In addition, untoward exercise responses need to be explained; thus the physical therapist needs a comprehensive understanding of how morbidity affects oxygen transport in general. In some instances, untoward exercise responses may alert the physical therapist to some previously undetected problem warranting referral to the patient’s physician. Oxygen transport can be affected by dysfunction of virtually any one of the major organ systems of the body (Box 6-1). The pulmonary and pleural complications of heart disease and the cardiac complications of pulmonary disease are usually predictable and therefore are most readily detected clinically. However, the cardiovascular and pulmonary complications of conditions affecting other organ systems can be more subtle, if not more devastating. The pulmonary complications of heart disease and the cardiac complications of pulmonary disease have been well documented for several decades.2 A mechanically inefficient heart disrupts the normal forward propulsion of deoxygenated and oxygenated blood to and from the lungs. Because the right and left sides of the heart function in series, a problem on one side inevitably has some effect, which can lead to a problem, on the other side. For these reasons, the heart and lungs should be thought of as a single functioning unit. Disruption of the cardiovascular and pulmonary circuit leads to the backlogging of blood and an increased volume of blood in the capacitance vessels or the veins. Right heart failure contributes to increased central venous pressure (i.e., right atrial pressure) and, if sufficiently severe, leads to bilateral peripheral edema in the dependent body parts. Because blood is not forwarded to the lungs adequately, hypoxemia can result. In turn, hypoxic vasoconstriction of the pulmonary circulation leads to increased pulmonary vascular resistance and, hence, to increased right ventricular afterload and work. In left heart failure, the normal pattern of increased ventilation to the bases may be reversed (i.e., the apices of the lungs may be better ventilated).3 If pulmonary edema complicates the clinical picture, the alveoli become flooded, resulting in reduced ventilation and ventilation and perfusion mismatching. The alveolar-arterial oxygen (A-aO2) gradient is then increased, diffusing capacity is decreased, and arterial partial pressure of oxygen (PaO2) is decreased. Lung compliance is inversely related to pulmonary artery pressures and interstitial fluid accumulation.4 The net effect of these abnormalities is both obstructive and restrictive pathophysiological patterns of lung dysfunction (i.e., reduced forced expiratory volumes and vital capacity and an overall increase in the work of breathing). Pleural complications can arise from lung disease as well as from heart disease. Both heart and lung function can be compromised by altered fluid balance in the pleurae. Fluid balance in the pleural space is comparable, in terms of its regulation, to that in the alveolar space. Both are determined by Starling forces. Specifically, hydrostatic pressure pushes fluid into the pleural space while oncotic pressure counters the effect of these forces. The net effect of these filtration and absorption forces is a minimal net filtration pressure. When the balance of these forces is disrupted, heart and lung function can be threatened. Excessive fluid floods the space, usually reflecting both excessive hydrostatic pressure and diminished oncotic pressure. The lymphatic vessels become overwhelmed and are unable to keep the pleural space dry. Pleural fluid accumulates and either displaces lung tissue (in small to moderate effusions) or restricts the opening of adjacent alveolar sacs, causing atelectasis (severe effusions),5 which, if sufficiently severe, may restrict cardiac filling. Pleural fluid accumulation poses a unique threat to oxygen transport as a result of its direct physical effect on the lungs, heart, or both, so it warrants special attention by physical therapists. Pulmonary lymphatics control fluid balance within the lung parenchyma. Lymphatic vessels arise within the pleurae and not within the alveolar capillary space. They drain fluid from the interlobular septae and subpleural areas into the hilar vessels and the primary tracheobronchial lymph nodes. Problems arising within the heart or lungs can contribute to imbalances in the major lymphatic inflow and outflow channels. This contributes to fluid accumulation, stagnation, and physical compression of the myocardium and lungs.6 Musculoskeletal conditions impact cardiovascular and pulmonary function secondary to their effects on muscle (in particular, the diaphragm, muscles of the chest wall, oropharynx, larynx, and abdomen) and bones and joints (e.g., arthritis, ankylosing spondylitis, kyphoscoliosis, and deformities secondary to neuromuscular diseases and chronic lung diseases) (Table 6-1). Additional effects are imposed by inactivity (i.e., muscle wasting, joint rigidity, and deformity). Increased joint rigidity limits the amount of physical activity a patient may perform, which contributes to cardiovascular and pulmonary compromise, in addition to the local effect of increased chest wall rigidity and compromised bucket-handle and pump-handle motions. The normal three-dimensional movement of the chest wall and normal pulmonary circulation and lymphatic function are compromised (Chapter 23). The cardiovascular and pulmonary manifestations of musculoskeletal conditions are summarized in Table 6-1. Table 6-1 Cardiovascular and Pulmonary Manifestations of Musculoskeletal Conditions The cardiovascular and pulmonary deficits associated with musculoskeletal disorders of the chest wall include reduced and potentially asymmetric lung volumes consistent with pulmonary restriction, reduced flow rates, reduced inspiratory and expiratory pressures, atelectasis, dynamic airway compression, ventilation-perfusion mismatching, inefficient breathing pattern, impaired cough and gag reflexes, increased risk for aspiration, increased risk for obstruction secondary to impaired mucociliary clearance, restricted mobility, compression of mediastinal structures and heart, and impaired lymphatic drainage, which depends on normal expiratory and inspiratory cycles.7 Osteoporotic fractures also compromise normal chest wall configuration, hence, pulmonary function.8 Autoimmune conditions that have musculoskeletal manifestations can be associated with systemic conditions. Rheumatoid arthritis, for example, is associated with accelerated atherosclerosis.9 Connective tissue, or collagen, vascular disorders (e.g., scleroderma, systemic lupus erythematosus, and systemic sclerosis) invariably affect the cardiovascular and pulmonary systems (Box 6-2).10 Inflammation and tissue injury can affect the airway, lung parenchyma, pulmonary vasculature, pleurae, respiratory muscles, heart, and pericardium. Shrinking-lung syndrome associated with chronic connective tissue changes is a feature of advanced disease and is characterized by loss of alveolar surface area, diffusion capacity, and lung volumes. Fibrotic changes increase the elasticity of the lung parenchyma and reduce lung compliance, thereby increasing the work of breathing. These changes are comparable to those in idiopathic pulmonary fibrosis. Both the electrical conduction system of the heart and its mechanical behavior are adversely affected by systemic connective tissue changes. Furthermore, connective tissue changes in the skin can lead to chest wall restriction. Renal-pulmonary syndrome has been associated with connective tissue conditions.11 The cardiovascular and pulmonary manifestations of connective tissue conditions are summarized in Box 6-2. Connective tissue conditions contribute to blood vessel changes and have been implicated in secondary pulmonary arterial hypertension.12,13 Cardiovascular and pulmonary consequences of neurological conditions reflect the pathophysiological mechanisms involved.14 There are three basic patterns of pathology: dysfunction of the central nervous system (CNS), dysfunction of the peripheral nervous system, and dysfunction of the autonomic nervous system. The cardiovascular and pulmonary manifestations of neurological conditions are summarized in Table 6-2. Table 6-2 Cardiovascular and Pulmonary Manifestations of Neurological Conditions
Cardiovascular and Pulmonary Manifestations of Systemic Conditions
Diagnosis and Assessment
Cardiovascular Conditions
Pulmonary Conditions
Musculoskeletal Conditions
Impairment
Factors that Potentially Contribute to Impairment
General Manifestations
Decreased alveolar ventilation
Altered respiratory mechanics
Chest wall rigidity
Decreased chest wall excursion
Impaired mucociliary transport
Airflow obstruction
Pulmonary restriction
Atelectasis
Inspissated secretions
Increased work of breathing
Inefficient breathing pattern
Increased work of the heart
Inefficient breathing pattern
Constrictive pericarditis
Decreased aerobic capacity
Manifestations in Specific Conditions
Rheumatoid arthritis
Pleuritis
Diffuse interstitial pneumonitis and fibrosis
Pulmonary arteritis
Bronchiolitis
Pleural effusions
Ankylosing spondylitis
Upper lobe fibrobullous disease
Chest wall restriction
Connective Tissue Conditions
Neurological Conditions
Manifestation Type
Impairment
Factors that Potentially Contribute to Impairment
General
Impaired mucociliary transport
Decreased physical mobility
Cilia dyskinesia
Increased mucus accumulation
Decreased cough and gag reflexes
Impaired airway protection
Increased airway resistance
Increased risk for airway obstruction
Impaired glottic closure
Increased risk for aspiration
Impaired alveolar ventilation
Decreased lung volumes and capacities, as well as flow rates
Weakness of pharyngeal and laryngeal structures
Respiratory muscle weakness
Decreased respiratory muscle endurance
Increased work of breathing
Decreased aerobic capacity and deconditioning
Specific
Multiple sclerosis
Respiratory muscle weakness
Impaired ventilation secondary to spasm
Increased oxygen consumption secondary to spasm
Increased oxygen consumption secondary to impaired posture and gait
Impaired gag and cough reflexes
Ineffective cough
Cerebral palsy
Increased oxygen consumption secondary to increased muscle tone
Decreased mobility and activity
Impaired movement economy
Impaired swallowing
Impaired saliva control
Decreased gag and cough reflexes
Impaired coordination of thoracic and abdominal motion during respiration
Ineffective cough and airway clearance mechanisms ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access