TEVAR for Type B Dissection with Malperfusion
JIP L. TOLENAAR, SANTI TRIMARCHI, and GILBERT R. UPCHURCH Jr
Presentation
A 49-year-old man, without significant past medical history, presented to an outside emergency room with complaints of acute onset of back pain, which he described as sharp and tearing in nature. The patient is in obvious distress and found to have a blood pressure of 190/85 mm Hg in both arms. Intravenous morphine results in some improvement of pain, but on physical examination, the patient has a mildly distended abdomen with diffuse tenderness to palpation. EKG shows no abnormalities. His laboratories are remarkable for a hematocrit of 38% and no troponin leak.
Differential Diagnosis
The differential diagnosis for a patient presenting with the acute onset of sharp or ripping back pain in a patient with hypertension includes: (1) myocardial infarction, (2) pulmonary embolism, (3) esophageal disease or reflux, and (4) aortic dissection.
Case Continued
The patient undergoes evaluation by CT scan imaging of the chest, abdomen, and pelvis, which demonstrates an acute type B aortic dissection from the left subclavian artery to the iliac arteries. The false lumen extends to the celiac access, and there is a mid-SMA filling defect described as thrombosis with retrograde filling (Fig. 1).
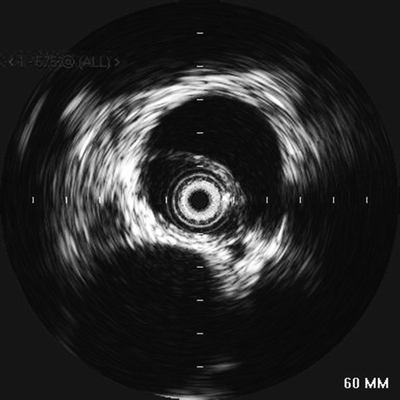
FIGURE 1 Intravascular ultrasound (IVUS) to confirm placement of the catheter in the true lumen. Image shows an evident intimal flap between the two lumens with the SMA coming off the false lumen at approximately 3:30.
Workup
CTA imaging is the preferred modality for diagnosis in type B aortic dissection and should be used liberally. Identification of the proximal and distal intimal tears is vital for the planning and success of the endovascular procedure. In addition, a transesophageal echocardiogram (TEE) was ordered, which confirmed a type B aortic dissection distal to the origin of the left subclavian artery. The echo also showed normal aortic valves and no wall motion abnormalities. The patient is started on a Nipride drip and transported through MedFlight to your institution.
Discussion
Acute aortic dissection is a life-threatening disease with an increasing incidence, due to aging population and improved imaging modalities. Aortic diseases are currently the 14th leading cause of death in the United States. Acute aortic dissection is defined as a laceration of the intimal layer allowing blood to flow along the medial layer, which will separate both layers of the aorta resulting in a true and false lumen. Prognosis of aortic dissection in untreated patients is poor, with a mortality of 20% to 30% before hospital admission and 50% within the first 48 hours. Important risk factors for aortic dissection include systemic hypertension, increasing age, atherosclerosis, bicuspid or unicommissural aortic valve, and connective tissue disorders (i.e., Marfan’s, Ehlers-Danlos, and Loeys-Dietz syndromes).
The most commonly used classification scheme is the Stanford classification, which makes a distinction between dissections that involve the ascending aorta (type A) and those that affect the descending aorta, distal to the subclavian artery (type B). In addition, further descriptions of dissections are based on the time from onset of complaint to diagnosis, so-called acute (less than 14 days) or chronic (more than 14 days).
Males are more commonly affected, typically at middle age. The majority of patients present with an abrupt onset of pain, tearing or ripping in nature and located in the chest and/or back. The disease course can be complicated by shock, cardiac tamponade, cardiac failure, myocardial ischemia/infarction, spinal cord ischemia, cerebrovascular accidents, mesenteric malperfusion, renal failure, pulse deficits, and limb ischemia.
Diagnosis and Treatment
The initial management of patients with aortic dissections should be directed at stabilizing the patient and prevent propagation and rupture of the aorta by adequate blood pressure control. Blood pressure should be maintained between 100 and 120 mm Hg systolic and ≤60 to 70 mm Hg diastolic, with a heart rate of less than 60 bpm. This medical therapy should be continued in acute B dissection, unless there is presence of complications, which require intervention. In patients presenting with malperfusion, endovascular interventions, including fenestration and stent and/or stent graft placement, are preferred as they convey less invasive and less morbid treatment in order to restore perfusion.
Two principal mechanisms may cause malperfusion, both requiring intervention. A static obstruction, which arises from a dissection flap that directly involves a side branch occluding the lumen, and is usually managed with stent placement into the branch vessel. The other possible cause of malperfusion is a dynamic obstruction, which may originate from obliteration of the side branches by the intimal flap and requires restoration of blood flow in the aortic true lumen and equilibration of blood pressure in both lumens. This usually can be established by covering the proximal entry tear with an aortic stent graft, in order to redirect blood flow to the true lumen, or by creating a hole (fenestration) in the intimal flap, allowing pressure equilibration and blood flow between the true and false lumen (Fig. 2).
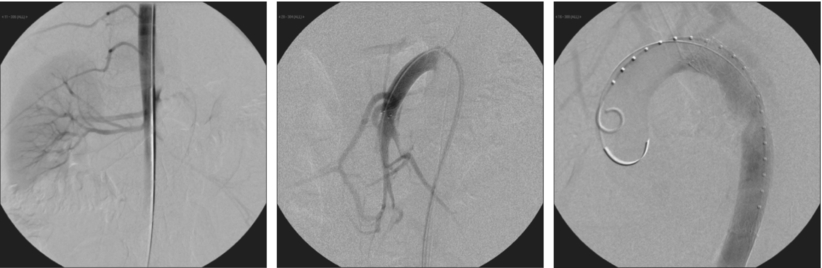
FIGURE 2 Angiography during TEVAR procedure. The first image clearly shows obstruction of the SMA and celiac trunk. The second image shows stent placement in the SMA with good distal perfusion. The third image documents stent graft placement in the thoracic aorta excluding the false lumen.



