(1)
Department of General surgery, Fuwai Hospital, Beijing, China
23.1 General Considerations
23.1.1 Embryology and Pathology
The diagnosis criteria, with regard to pathology and anatomy, of tetralogy of Fallot (TOF) include (1) the aorta overrides on both the left and the right chambers, when the level of the AV orifice is heaved higher (Fig. 23.11a–c); (2) subaortic VSD is present; (3) right ventricular infundibulum stenosis or complication with PV stenosis is present; (4) fibrous connections remain between the aortic and mitral valves, when the aortic and tricuspid valves are joined with fiber or separated by muscle; and (5) right ventricular hypertrophy is present.
The embryological basis of TOF is malformation of the conotruncus:
1.
The rotation of the conotruncus is not adequate. The aorta is not completely connected to the LV. It is biventricular in origin and overrides on the ventricular septum, which is a result of the AV not adequately moving to the left, posterior, and inferior of the PV from the position at the early embryonic stage, when both valves are at the same level as the ventricles (AV is on the right, and PV is on the left).
2.
The conotruncus does not divide equally, and the pulmonary artery is smaller than the aorta (the same size at normal condition).
3.
The conal septum does not join with the membranous ventricular septum and the muscular ventricular septum to close the interventricular foramen, and a subaortic VSD is in position. The subpulmonary infundibular hypoplasia, the right ventricular infundibulum shortening and stenosis, and the inadequate rotation of the conotruncus lead to the subaortic VSD and the aorta overriding. During the fetus period, the pressures of the right and left ventricles are equal, and the right ventricular hypertrophy is acquired.
23.2 Key Points in the Anatomy
In the normal condition, a longitudinal line through the midpoint of the anterior mitral leaflet, termed midline of mitral valve, always goes across the commissure of the left coronary aortic cusp and noncoronary cusp. In TOF, the commissure displaces rightward and leaves the midline when the aorta rotates rightward and anteriorly. With regard to the relationship between the membranous septum and AV, the membranous septum is immediately below the commissure of the right coronary aortic cusp and the noncoronary cusp. In the case of TOF, the commissure rotates rightward and anteriorly, and the septum is below the noncoronary cusp.
The aortic annulus is lifted higher: the VSD is subaortic and equal to an intracristal VSD, the inferior edge of which is formed by a muscular septum and a septal band. The aortic annulus overrides rightward and forms the superior edge of the VSD when the anterior edge is the anteriorly displaced parietal band. Hence, the VSD in TOF might be considered an infundibular VSD.
23.2.1 Features of the VSD (Figs. 23.12a–j, 23.13a–d, and 23.7a–d)
The superior edge of the VSD is the connection portion of the aortic annulus and the front wall of the RV, which is equal to the inferior of the right coronary aortic cusp or the noncoronary cusp or both. In all the specimens, this edge is composed of muscular tissue, and the anterior end of the muscular edge extends to the parietal band when the posterior end is near the TV annulus.
The anterior edge is the anteriorly displaced parietal end of the crista supraventricularis, which arises from the front portion and extends to the free wall of the RV to form the posterior border of the RVOT. This edge is composed of muscular tissue in all the specimens.
The posterior edge descends from the aortic annulus, then crosses the front of the TV annulus and reaches to the posterior end of the ventricular septum. It is actually part of the RV free wall in front of the TV annulus. The muscle bundle (or fiber bundle) of the edge is equal to the posterior limb of the parietal band. Intracristal VSDs occur commonly in TOF, but occasionally, the VSD extends to the membranous septum.
From the surgical point, the posterior edge of the VSD is very important because it is near the atrioventricular bundle. Based on the strategy of the operation, VSDs in TOF might be divided into two types:
1.
There is no muscular bundle between the posterior edge of the VSD and the TV annulus, and the posterior edge is composed of the fibrous connections between the tricuspid and the aortic annuli. With the malformation of the membranous septum, the atrioventricular bundle passes along the border of the membranous septum, which should be avoided during the repair of the VSD.
2.
There are muscular bundles between the posterior edge of the VSD and the TV annulus, and muscles surround the VSD completely; therefore, the aorta is separated from the tricuspid annulus. A well-developed membranous septum is located behind the muscular bundle, in which case there is no conduction bundle, and it is safe to sew the rim of the muscles directly during repair. The others belong to the intermediate type.
23.2.2 Features of Pulmonary Artery Stenosis
The infundibular stenosis is sometimes associated with PV stenosis and stenosis of the pulmonary artery trunk or the branches (Fig. 23.8a–d). The stenosis of the valve might be a result of bicuspid or commissural fusion. Because of the infundibular stenosis, dilation of the pulmonary artery after the stenosis is rarely seen in clinic, and the diameter of the pulmonary trunk is smaller than that of the ascending aorta. Because different surgical strategies are selected based on the type and extent of the infundibular stenosis, it is important that these factors be estimated preoperatively:
1.
The stenosis is confined and located at the proximal part of the infundibulum, forming a “third ventricle.” With a well-developed pulmonary annulus, the RVOT obstruction can be relieved simply by excising the hypertrophic crista supraventricularis (Fig. 23.5a–c).
2.
3.
In the malformation or the absence of the infundibulum, a valve conduit is commonly needed to link the RV to the pulmonary trunk to correct the pulmonary valvular stenosis or the pseudotruncus arteriosus due to pulmonary valve atresia (Fig. 23.24a–f). As the burden increases after birth, the patients’ RV becomes increasingly hypertrophied, which adds to the RVOT obstruction. As the coronary arteries on the surface of the RV become larger, tortuous, and dilated, making an incision into the RV safely becomes more difficult.
23.3 Technical Risks with Repair of TOF
The basic principles of repair of TOF are to relieve the ROVT obstruction and the PV stenosis and to repair the VSD (Figs. 23.19a–f, 23.9a–e, 23.16a–f, 23.21a–f, 23.3a–e, 23.4, and 23.1). The most common risks include (Fig. 23.17a–e):
1.
There are often many aortopulmonary collateral arteries (APCAs) in adult patients. If this condition is associated with patent ductus arteriosus, a large amount of blood flow can drain into the pulmonary artery, even after an aorta clamp is placed, which not only affects the view of the surgery field but also causes destruction of the blood.
2.
Large coronary artery branches on the surface of the RV can be damaged when the right ventriculotomy is made, which might cause localized myocardial infarction (Fig. 23.23a–e).
3.
Residual RVOT obstruction may occur. One reason is that the RVOT muscular obstruction is inadequately relieved, and the other is that repairing the subaortic VSD might induce RVOT obstruction, requiring that the RVOT be widened by using a patch.
4.
Residual pulmonary artery and RVOT obstructions may occur, usually the result of not being adequately widened by using a patch (Fig. 23.8a–d).
5.
PV regurgitation and RV dysfunction may result. With hypoplasia PV, enlarging a pulmonary annulus might cause severe regurgitation, so the PV homograft is recommended.
6.
7.
Damage to the AV may occur. The AV might be injured during resection of the RVOT muscles and repair of the VSD, resulting in severe regurgitation.
23.4 Specimen Demonstrations
23.4.1 Typical Postoperative Specimen of TOF Showing the Override of the Aortic Valve
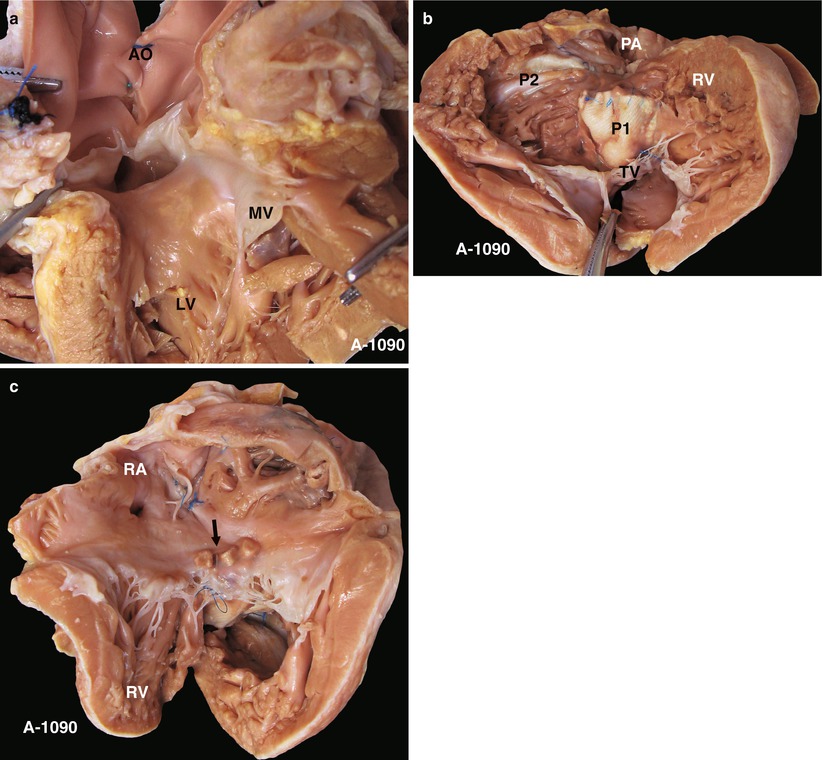
Fig. 23.1
(a) Specimen 1090. TOF. Viewed from the LV, two cusps of the AV can be seen to override across the VSD, which infers a severe 70 % override. However, the AV retains fibrous continuity with the MV, which distinguishes this case from the DORV. AO aorta, LV left ventricle, MV mitral valve. (b) Specimen 1090. Viewed from the front side of the heart, the large VSD patch (P1) is seen adjacent to the area above of the crista supraventricularis. The “P2” marks the RVOT expanding patch. The muscular bands of the RVOT are transected. RV right ventricle, TV tricuspid valve. (c) Specimen 1090. The arrow shows the correct position of the reinforcement pledgets used to patch the VSD via the RA
23.4.2 TOF, the VSD Patch Removed to Show the Stitched Edge of the AV and the VSD
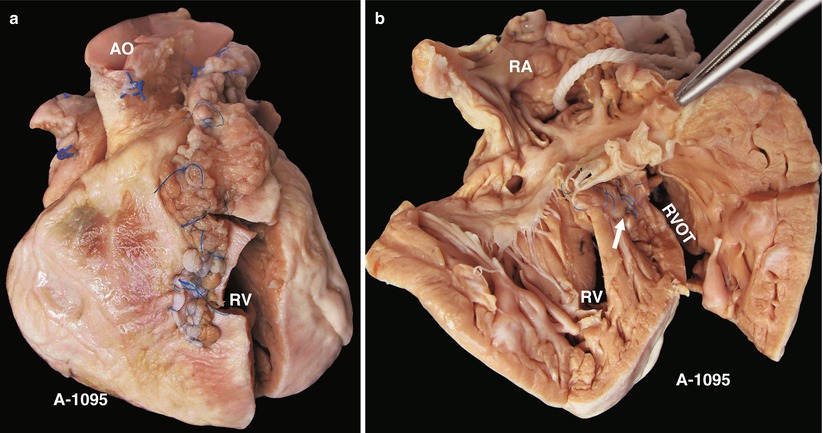
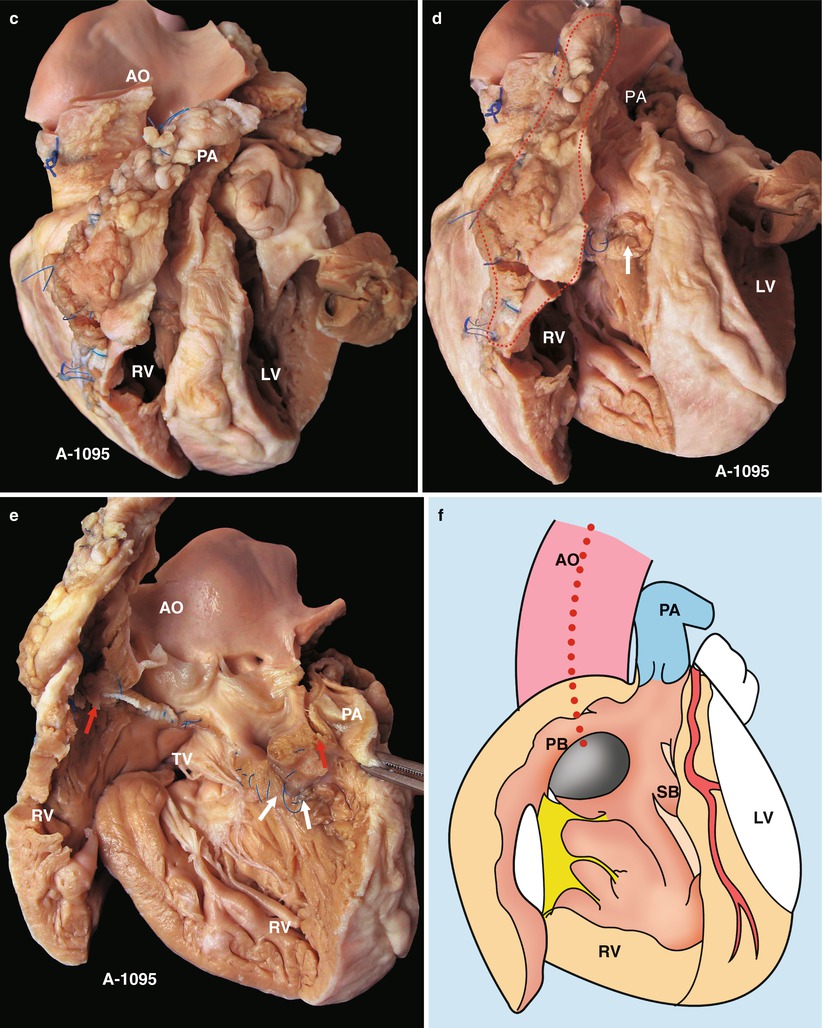
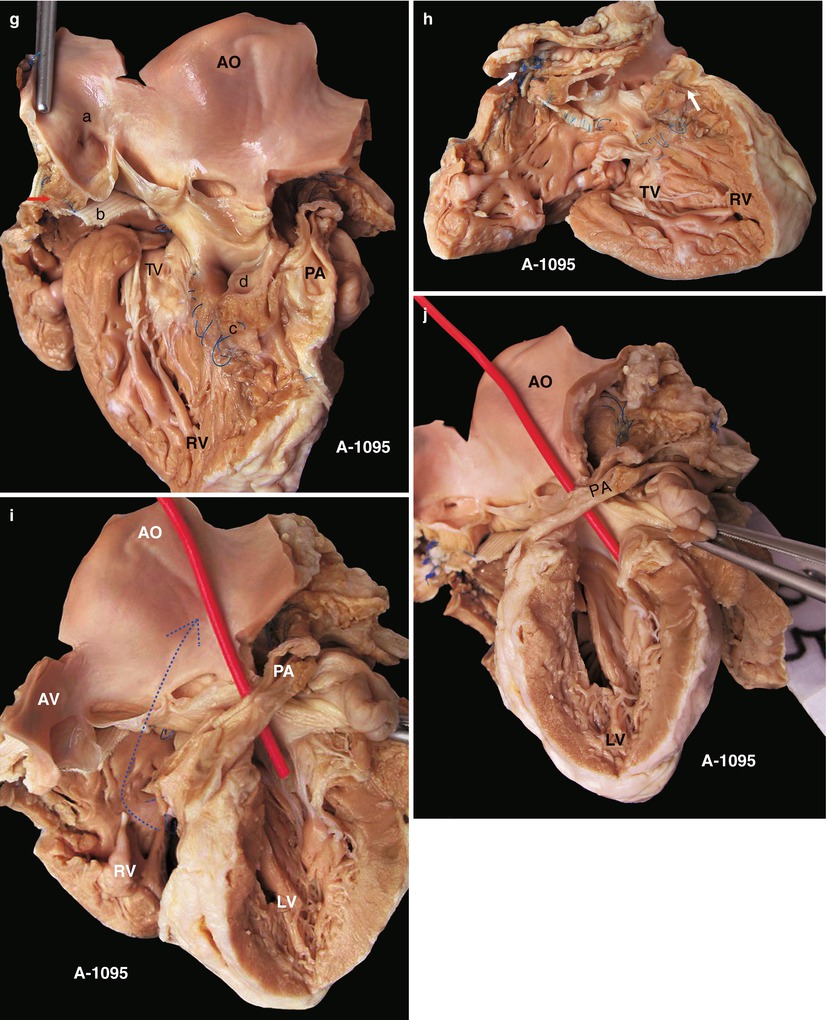
Fig. 23.2
(a) Specimen 1095. External view of the heart after repair of TOF. The RVOT is enlarged by a transannular patch, and the RVOT and the PV annulus are exposed through an incision carried along the ventricular septum. AO aorta, RV right ventricle. (b) Specimen 1095. The RA (RA), RV (RV), and TV can be seen simultaneously through the RVOT. The VSD patch is removed, but the sutures of the inferior rim of the VSD remain. The arrow points at the blue sutures, among which one entangles the chordae tendineae of the TV. The “RVOT” shows the narrowing of the RVOT. (c) Specimen 1095. The right ventricle and the left ventricle cut open along both sides of the ventricular septum. The transannular patch crossing from the RVOT to the PA is totally demonstrated. Meanwhile, the aorta (AO) enlargement and the pulmonary artery (PA) stenosis also can be seen. (d) Specimen 1095. The dash line shows the border of the patch from the RV to the pulmonary artery, and the arrow indicates the transected muscles of the parietal band. (e) Specimen 1095. The relationship between the aortic valve and the rim of the ventricular septal defect. Both the front wall of the RV and the ascending aorta are incised and then raised above to fully expose the override of the AV and the exact position of the stitched edge of the VSD patch. The red arrow indicates the upper edge of the patch. Residual polyester patch remains in front of the left red arrow, which indicates that the AV may be wounded here by the suture. The white arrows indicate the lower edge of the VSD patch. (f) Specimen 1095. Diagram of Fig. 23.12e. The dashed line indicates the incision. PB parietal band, SB septal band. (g) Specimen 1095. Coronal plane view of the heart to expose the override of the aortic valve and the stitched edge of the ventricle septal defect patch. The red arrow denotes that the AV might be wounded here easily by the suture. a orifice of coronary artery, b residual patch of the VSD, c residual sutures on the lower edge of the VSD, d VSD. (h) Specimen 1095. The image shows that the RVOT, especially the muscles of parietal band (arrow), lies adjacent to the AV leaflets, which can be injured during resection of the hypertrophied muscles of the RVOT. (i) Specimen 1095. Demonstration of the override of the aorta and direction of the right-to-left shunt. The blue dashed line shows that the blood flow is drained from the RV into the aorta, and the red bougie represents the LVOT. (j) Specimen 1095. Although two leaflets of the AV override onto the RV, there are fibrous connections between the aortic and mitral valves. Hence, the case is diagnosed as TOF rather than DORV
23.4.3 View of Intact VSD in TOF
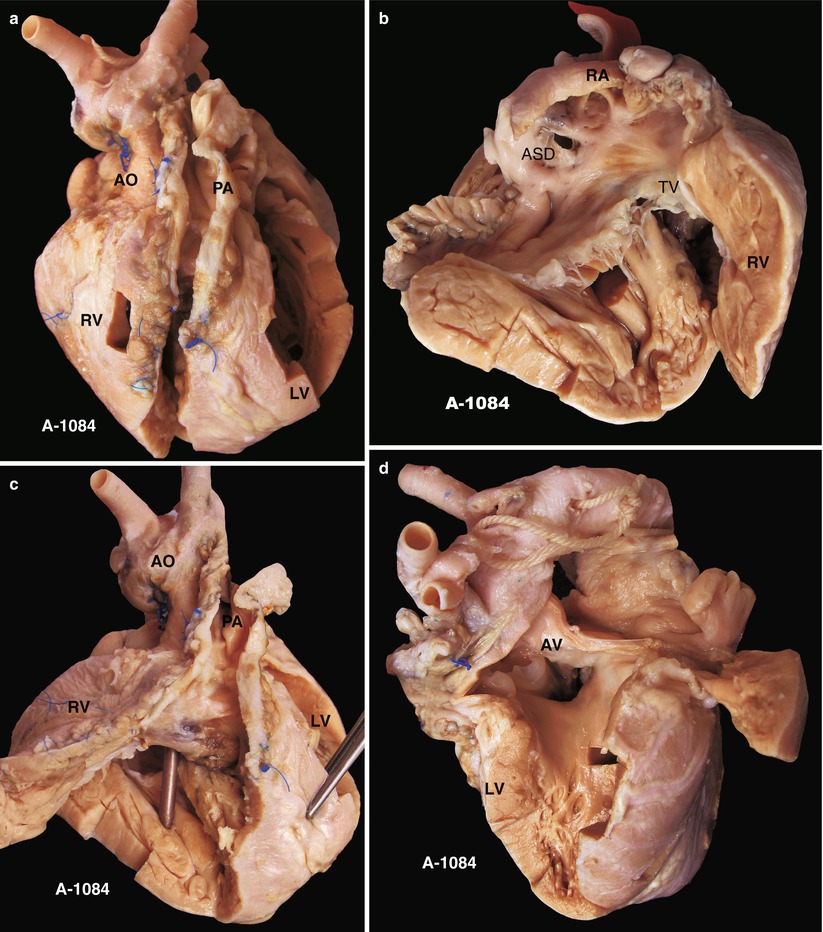
Fig. 23.3
(a) Specimen 1084. Specimen of postoperative TOF. The VSD patch is removed during the autopsy to show an overview of the VSD. This is a front view of the specimen. AO aorta, LF left ventricle, PA pulmonary artery, RV right ventricle. (b) Specimen 1084. An ASD is accompanied. The RV is obviously hypertrophied. RA right atrium, TV tricuspid valve. (c) Specimen 1084. The patch expanding the front wall of the RV is cut open to reveal the VSD below the crista supraventricularis. Remnant polyester pledgets remain on the edge of the VSD, but the patch is removed. The bougie is conducted across the VSD into the RV from the ascending aorta. (d) Specimen 1084. The VSD and the overriding aortic valve viewed from the left ventricle side. There is no trace indicating a repair on the left side of the VSD
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


