Chapter 90
Technique
Endovascular Aneurysm Repair
W. Charles Sternbergh III,
In 1991, Juan C. Parodi published his seminal work on using a homemade tube endograft to treat an abdominal aortic aneurysm (AAA).1 Although there had been previous experimental studies published on this topic,2,3 Dr. Parodi’s first-in human report was the accelerant that fueled subsequent innovation in the field of endograft technology. Within 5 years, industry-made endografts began to undergo clinical trials in the United States4 culminating in the first commercially available devices for endovascular aneurysm repair (EVAR) in September 1999.5,6 In Europe and Australia, aortic endografts were available earlier because of differences in governmental regulatory oversight. Second and third generation endografts came to market in subsequent years and provided improvements in fixation, sizing versatility, and delivery profile.7–9 All devices underwent further refinements after initial release, improving both long-term outcome and applicability of EVAR. Endografts for treatment of thoracic aortic aneurysm (TEVAR) began clinical trials at the end of the 20th century, culminating in the first U.S. commercially available device in 2005.
In this short time-span of 10 to 15 years, the treatment paradigms for aortic pathology have undergone a seismic shift. Endovascular repair has become the preferred treatment modality for the majority of abdominal and thoracic aortic aneurysms. Other thoracic pathology, including traumatic transaction and type B dissection with malperfusion, are also now preferentially being treated with endografts. Endovascular aneurysm repair has demonstrated improvements in perioperative mortality and major morbidity in both infrarenal10–12 and thoracic locations13,14 compared with traditional open repair. Such improvements in perioperative safety and its minimally invasive nature have been the primary drivers of these remarkable changes in practice patterns.
Endograft Configurations
Chapter 95 provides detailed descriptions of specific endograft designs. Bifurcated endografts are currently utilized in greater than 95% of EVAR cases. These are either modular (Endurant, Medtronic, Minneapolis, Minn; Excluder, W. L. Gore, Flagstaff, Ariz; Ovation, TriVascular, Santa Rosa, Calif; Zenith, Cook Medical, Bloomington, Ind) or unibody (AFX, Endologic, Irvine, Calif). Early endograft designs utilizing a tube endograft (EVT/Ancure, Guidant, Indianapolis, Ind) had very limited applicability and poor long-term results, making this design undesirable for fusiform infrarenal aneurysms.15 In the infrarenal position, tube endografts are now utilized only for focal saccular aneurysms or penetrating ulcers that have sufficient normal distal aorta for a dependable seal. Although there are no commercially available devices for this indication, off-label use of “stacked” aortic cuffs,16 larger iliac components, or small diameter thoracic extensions can be utilized for this purpose. Aorto-uni-iliac endografts (ReNu, Cook Medical) can be utilized in conjunction with a contralateral iliac occlusion device and a femoral-femoral bypass. The relative indications for use of this configuration are listed in Box 90-1. Branched and fenestrated endografts can be utilized in juxtarenal and/or pararenal aneurysms and thoracic AAAs where there is an inadequate normal aorta to achieve a durable seal adjacent to a critical side branch (see Chapter 91).
Preoperative Sizing and Planning
It is axiomatic that precise sizing and preoperative planning are essential for successful initial and long-term outcome after EVAR. Failure to pay close attention to these critical details will predictably increase the risk of a poor outcome. All patients require a custom-sized device that conforms to their particular anatomy. It is incumbent on the treating physician to master a comprehensive understanding of the nuances of precise endograft sizing. These tasks should not be delegated to other support staff or device representatives.
Preoperative Imaging
Computed Tomography Arteriography
Computed tomography arteriography (CTA) is the cornerstone of preoperative imaging for EVAR. The maximum recommended slice diameter is 2.5 mm for standard devices, whereas sizing for fenestrated or branched devices requires 1 mm or less cuts. Intravenous contrast should be routine unless the patient has severe renal insufficiency. The axial, coronal, sagittal, and three-dimensional reconstructions (Fig. 90-1A) should all be reviewed. Postprocessing software allows further image interrogation by generating centerline reconstructions. This allows for more precise length and diameter measurements, which are essential with custom fenestrated devices. Depending on institutional capabilities, these reformatted images may be routinely available. Access to dedicated work stations that allow the end user to personally postprocess and manipulate the images is ideal. Alternately, such image processing is available by third parties.
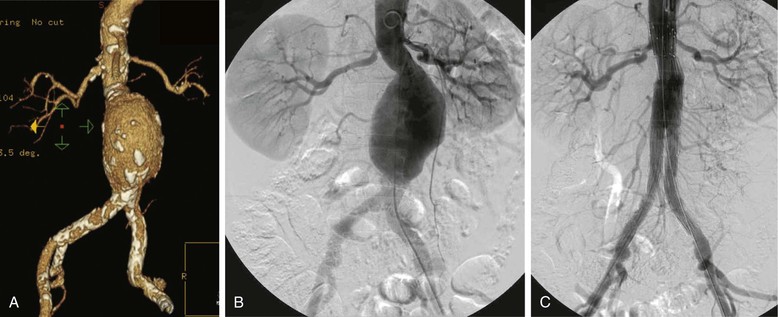
Figure 90-1 A, Computed tomographic angiography reconstruction of a 6.3-cm abdominal aortic aneurysm (AAA). B, Angiogram demonstrating the same AAA. C, Immediate postendovascular aneurysm repair angiogram demonstrating exclusion of the AAA without endoleak.
Standard digital subtraction arteriography (DSA) has little utility in the preoperative evaluation for EVAR and should not be routinely employed. With rare exception, all anatomic details can be obtained with CTA scanning.
Patients with significant renal insufficiency present challenges in preoperative imaging before EVAR. Frequently, noncontrast CT scanning provides enough anatomic information to proceed with standard EVAR. Diameter and length measurements can be made from such a study, and if the anatomy appears uncomplicated, and if there is no clinical suspicion of associated occlusive disease, it may be reasonable to proceed. However, potentially important anatomic information can be missed: (1) potential presence of laminated thrombus in the aortic neck; (2) patency of important side branches, such as the hypogastrics: and (3) potential occlusive disease in the common or external iliac arteries. Significant calcification in the iliac arteries, particularly the external iliacs, should make the operator suspicious that associated occlusive disease may be present. All of these anatomic details may negatively affect the performance of EVAR. Finally, if contrast is absolutely necessary, a pigtail catheter can be placed in the perirenal aorta and used to inject diluted contrast while performing CT scanning. This reduces the amount of contrast utilized by approximately 50% to 75% compared with that required for intravenous administration.
Alternative Imaging
In the patient with severe renal insufficiency who needs better definition of pertinent anatomy before EVAR, other options to CT are available. Intravascular ultrasound (IVUS) can be used to size the aortic and iliac seal zones, evaluate for potential aortic neck eccentric thrombus, and interrogate the external iliacs for potential occlusive disease. Direct angiographic imaging can be obtained using carbon dioxide as the contrast agent with relatively good visualization. Until recently, gadolinium was also an option as an alternate contrast agent. However, the association of gadolinium use with subsequent renal impairment in patients with known significant renal insufficiency has made its use relatively contraindicated.
Endograft Sizing
Aortic Neck Diameter
Measurement of the aortic neck diameter should be taken at the level of the lowest renal artery and 15 mm caudal. These measurements should be made from the minor axis of axial cuts or from reformatted slices that allow a plane perpendicular to the centerline. The key here is not to overestimate diameter based on tortuosity that is frequently present in the aortic neck. Using electronic calipers, these measurements are generally made from adventitia to adventitia to size the aortic neck for most devices. Intima-to-intima measurements were used in the U.S. pivotal studies of the Excluder endograft and are recommended for use with this device.
Endografts should be oversized 10% to 20% in comparison to the aortic neck. In practical terms, this usually translates into a 3 to 4 mm larger endograft than the aortic neck. Currently, EVAR devices range from 20- to 36-mm diameters and can accommodate aortic diameters of 18 to 32 mm. TEVAR devices currently range from 21 to 45 mm and can accommodate treatment diameters of 16 to 42 mm.
Failure to adhere to precise sizing guidelines will predictably increase the risk of both immediate- and long-term failure. The dangers of inadequate oversizing is intuitive; if there is no full apposition of the endograft to the aortic wall, the risk of a type I endoleak is substantial. The risks of excessive oversizing are less obvious, however. Bench testing has demonstrated that oversizing more than 20% creates pleats in the fabric (Fig. 90-2). This pleating of the fabric may contribute to an increased risk of a type I endoleak, and possibly decreased fixation. The deleterious effects of excessive device oversizing for EVAR are not device-specific. In a single series study with the AneuRx device (Medtronic), oversizing greater than 20% was associated with an increase in late aortic neck dilation and device migration.17 In a multicenter study utilizing the Zenith endograft, oversizing more than 30% was associated with a marked increase in the risk of aneurysm expansion and migration.18 No effects on late aortic neck dilation were observed.
Sizing the Conical Aortic Neck
Patients with conical necks (those with more than 2 to 3 mm change over the first 15-mm length of aortic length) pose an interesting sizing conundrum. In a patient with an aortic neck diameter of 20 mm at the renal arteries that dilates to 24 mm at 15 mm caudal, sizing to the larger diameter would suggest the use of a 28-mm endograft. However, that corresponds to a proximal oversizing of 40%. Conversely, if sized according to the smaller diameter (a 24-mm endograft in a 20-mm neck), there would be inadequate oversizing in the caudal larger area. In situations such as these, it is prudent to split the difference to give at least a minimum of 10% oversizing in the larger segment, and a less than 30% oversizing in the smaller segment. If the degree of size mismatch does not allow such sizing (>3-4 mm conical change in first 15 mm of neck), EVAR is ill-advised.
Length Measurements
Making accurate length measurements between proximal and distal landing zones is critical in choosing the correct endograft components. For EVAR cases, counting the axial cuts is very accurate when calculating the distance between the lowest renal artery and the aortic bifurcation in the absence of significant tortuosity or aortic neck angulation. Axial measurements will underestimate the length between the aortic bifurcation and the hypogastric arteries, especially with very tortuous vessels. Conversely, length measurements based on centerline calculations with EVAR will frequently overestimate the true length needed. In TEVAR cases that include the aortic arch, axial cuts are not nearly as helpful, and even centerline reconstruction measurements can be inaccurate, usually underestimating the true length. If more than one thoracic component is employed, a minimum of 5-cm overlap is needed. This component overlap should be even greater if the bridge area is within a large aneurysm without significant laminated thrombus, or in an area with significant angulation. Ultimately, knowledge of these imaging limitations allows experienced operators to use their geshalt in choosing the final appropriate length.
Situations that typically require longer iliac limbs than the measurements suggest include extreme iliac tortuosity, “balleting” of the limbs (Endurant and Excluder) (Fig. 90-3), and the need to extend to the external iliac arteries. It these anatomic circumstances, it is prudent to choose a longer length when in doubt. Modular devices will also allow some degree of flexibility at the time of implant by adjusting the amount of overlap between the components.
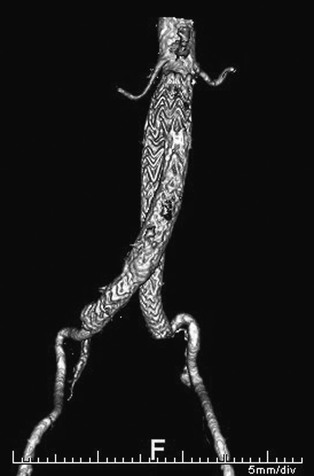
Figure 90-3 “Balleting” of iliac limbs. This technique can facilitate cannulation of the contralateral gate of short-bodied modular endografts. A longer iliac limb is frequently required.
Modular devices with variable body lengths (Zenith) give additional flexibility in sizing. With this device, the contralateral iliac gate is ideally positioned 1 to 2 cm from the aortic bifurcation. Unlike the iliac components, when in doubt about the correct body length, choose the shorter length. Other scenarios that call for a shorter body length include patients with a small (<20 mm) distal aortic neck or those with an eccentric calcific “reef ” in the terminal aorta. In these instances, after opening the ipsilateral limb (12-mm diameter), there may be inadequate room for the contralateral gate to open, making cannulation difficult.
Iliac Diameters
Limbs for the iliac arteries should be sized 10% to 20% greater than their minor axis diameter. For nonectatic iliac arteries, this generally translates into an iliac limb diameter 1 to 3 mm larger than the vessel. Particular care should be made to correctly size the iliac limb if extension to the external iliac is required. Excessive oversizing may increase the risk of limb thrombosis.
Patient Selection
The major anatomic factor in predicting suitability for EVAR is the character of the aortic neck. Length, diameter, angulation, and shape of the aortic neck are all important factors. The instructions for use (IFU) of most current devices suggest a minimum neck length of 10 to 15 mm and angulation of less than 45 to 60 degrees. Taking into account proper oversizing and available endograft sizes, aortic diameters up to 32 mm can be accommodated. The shape of the neck is also important in both immediate- and long-term success. The ideal is a parallel neck without any eccentric laminated thrombus. Patients with irregular-shaped necks will have a greater risk of an inadequate seal. In addition to patients with conical and reverse conical necks, the occasional patient will have a localized posterior bulge in the neck (“double-bubble”) that can compromise the seal zone. Treatment of anatomically compromised necks can negatively affect both short- and long-term results of EVAR.
How far can these aortic neck constraints be pushed? Although a patient with a single adverse aortic anatomic variable can frequently be treated effectively, those patients who have aortic necks with multiple compromising issues are more problematic. Although highly angulated necks are associated with inferior outcomes,19 if the neck length is more than 2 cm and the shape of the neck is parallel, the patient may be a reasonable EVAR candidate. Patients with neck lengths of 10 to 15 mm can frequently attain a good initial outcome in the absence of other anatomic compromise. However, the risk of late neck degeneration and a subsequent type IA endoleak is likely increased, particularly in the setting of a larger aortic neck diameter. All devices will have compromised outcomes if the anatomic constraints are profoundly exceeded.20–22 Patients with aortic necks less than 10 mm in length should be considered for either an open repair or EVAR with adjunctive endovascular techniques (fenestrations, branches, snorkel; see Chapter 91).
Anesthesia, Access, and Imaging
Anesthesia
The choice of anesthetic technique can be tailored to the patient’s comorbidites and body habitus. In all situations it is ideal to be able to control the patients’ respirations, either by asking them to hold their breath or doing it manually with the respirator. With open vascular access, a regional (spinal) or general anesthetic is favored by most operators. Although local infiltration and intravenous sedation is possible in such scenarios, it is frequently challenging to keep the patient comfortable. Frequently, a substantial amount of intravenous sedation becomes necessary. The diminished alertness of the patient makes it difficult for effective breath-holding, potentially compromising the precision of the proximal endograft placement. A regional anesthetic is the ideal choice in a patient with severe pulmonary disease in whom avoidance of endotrachial intubation is desirable. When such an anesthetic is employed, only light intravenous sedation in used so that patients can hold their breath. In a patient who may need an iliofemoral conduit, general anesthesia is clearly the first choice.
Vascular Access
Access to the femoral vessels can be done in an open or percutaneous fashion.
Open
With open access, either a vertical or oblique skin incision can be utilized. The advantages of a vertical incision include the ease of gaining additional exposure to the femoral vessel. If preoperative imaging suggests significant femoral artery occlusive disease that might require an extended endarterectomy and patch angioplasty, this may be the preferred incision. Postoperative wound problems can be minimized by keeping the incision above the femoral crease, which is possible in most patients. The oblique skin exposure is favored by many operators because wound problems may be less frequent. After the superficial fat is divided in the plane parallel to the oblique skin incision, vertically oriented deeper dissection is then performed. This open exposure may be particularly useful in morbidly obese patients. If the angle into the femoral vessel is too acute, a small counter incision can be made inferior to the skin incision, just large enough for the needed sheaths.
Percutaneous
The popularity of percutaneous access for EVAR and TEVAR has increased over the last 5 years. A recent systematic review of percutaneous access for EVAR cites the potential for a shorter procedure time, less postoperative pain, and a lowered risk of wound issues.23 Disadvantages include increased hospital cost and the occasional need for urgent conversion to open repair. Access is possible percutaneously for sheath sizes up to 24F. The reported success rates for percutaneous closure of ≥18 to 20F sheaths are 78% to 95%, increasing to as high as 95% to 99% with sheaths of 12 to 16F.24,25 A substantial learning curve for the technique has been observed.26 Because of the 5% to 10% rate of failure in closure of larger arteriotomies, immediate availability of surgical closure is essential. Prudent patient selection and careful study of the preoperative imaging of the femoral arteries will maximize the success rate of percutaneous access for EVAR. Box 90-2 lists relative contraindications for this procedure. Preoperative imaging should be closely scrutinized for anatomic information regarding the common femoral artery, including its diameter, associated occlusive disease (particularly anterior calcification), and the location of the femoral bifurcation relative to the inguinal ligament.
This technique utilizes off-label use of suture-mediated closure devices in a “pre-close” fashion. The common femoral artery is ideally accessed 1 to 2 cm proximal to its bifurcation but below the inguinal ligament. Some operators routinely employ a micropuncture set with a 21-gauge needle and 3F introducer rather than a standard 18-gauge needle. Routine use of ultrasound guidance has been recommended to increase the accuracy of puncture placement.27 Verification of the wire entry location should be routine, done through a small retrograde arteriogram with the appropriate ipsilateral oblique to better define the superficial femoral artery (SFA) and/or profunda origins.
A 0.035-inch guidewire is then advanced into the aorta and a 7F sheath placed. The “preclose” technique may be performed with either a single 10F Prostar or two 6F Proglide devices (Abbott Laboratories, Abbott Park, Ill). Utilizing the Proglide devices,24 the first device is deployed with a 30-degree medial rotation. Maintaining wire access, the second device is deployed with a 30-degree lateral rotation. These devices place a single monofilament suture proximal and distal to the puncture site. These sutures are exteriorized as the delivery device is removed and then tagged for later use. The 7F sheath is then replaced for hemostasis. Alternately, a single 10F Prostar device may be employed.25 Some operators have suggested the routine use of serial “coons” dilators to predilate the tract and arteriotomy after the “preclose” sutures are placed, but before delivery of the sheath for endograft delivery. This maneuver may decrease the chance of the “lip” of the dilator and/or sheath interface catching on the subcutaneous tissue or the arteriotomy itself.
After completion of the EVAR, the delivery sheath is removed while retaining guidewire access. Hemostasis is maintained with manual compression until the preformed knots (Proglide) are cinched down with the knot pusher. After hemostasis is confirmed, the guidewire is removed and manual compression is reapplied. Reversal of heparin with protamine is suggested at this time. If there is pulsatile bleeding after the two Proglide sutures are tied, a third Proglide can be placed over the existing wire and deployed. If there is continued pulsatile bleeding, an open surgical repair should be performed. Replacement of an appropriate sized dilator over the existing wire facilitates this exposure by providing a nonhemorrhagic field.
Iliac Occlusive Disease
The iliac vessels should be carefully studied preoperatively with CTA so any potential difficulties in access can be anticipated. Angioplasty can be used for focal iliac occlusive disease immediately before endograft insertion. Bare metal stents should not be initially placed because the large sheath for the endograft will need to be placed through them, increasing the possibility of stent migration. If an iliac lesion warrants a stent placement and is not covered by the endograft, place the stent at the completion of the EVAR. Hydrophilic coons dilators can be effective in dilating longer segments of occlusive disease, and can also act as a guide as to whether the endograft sheath will pass. These should only be placed over very stiff wires. A word of caution, however, is in order here. Sometimes, a larger sheath can be negotiated into the aorta with significant pressure, only to have catastrophic bleeding from iliac disruption upon removal of the sheath or with repeated placement. This reality should be considered when planning the repair to minimize the need for repeat traversal of a diseased iliac artery.
There are other potential endovascular approaches to obtaining access in the setting of small iliac arteries. It is possible to create an “internal endo-conduit” by placing a covered stent in the external iliac vessel, which then undergoes aggressive angioplasty to the needed diameter of 9 to 12 mm.28,29 The covered stent will be protective of the extensive dissection and/or free rupture of the native vessel caused by the aggressive angioplasty. To be safe, there must be a sufficient proximal and distal seal zone for the covered stent. Such landing zones are not routinely present, however. Prudent patient selection is critical. Although these techniques have been anecdotally reported with successful outcomes,29 larger series will be needed to establish the safety of these innovative access procedures before broader utilization.
Recently, a balloon-expandable sheath has become available to help in accessing smaller iliac arteries (SoloPath, Onset Medical Corp, Irvine, California). This sheath is delivered in a 14F size, and then dilated by an integrated balloon to sizes up to 24F. Its use has been reported to be successful in a small series of patients.30 When removing the sheath, it is prudent to maintain wire access and perform an iliac arteriogram; treatment of secondary vessel injury may occasionally be needed with bare or covered stents.
Iliac Conduit Placement
The potential use of a surgically placed conduit should be in the “toolbox” of all operators performing endovascular aortic aneurysm repair.31 The need for a conduit for EVAR is now unusual, particularly with the introduction of lower profile devices. However, the larger French sizes for TEVAR and the higher prevalence of women needing this procedure have translated into a 10% to 20% usage in most larger series. Patients with diffusedly calcified and small external iliac arteries clearly need a conduit most of the time. If the operator is concerned about access, serious consideration should be made to perform a conduit without trying to access the iliac artery. Visual inspection of the common femoral and terminal external iliac arteries with open exposure can help in making this decision in equivocal cases. The worst time to perform a conduit is after the iliac vessel has been injured; the potential morbidity and mortality rates rise considerably.
An iliac conduit is performed through a right or left low retroperitoneal incision. Staying in the retroperitoneal plane, the common iliac artery is readily identified. We favor a standard end-to-side anastamosis with the distal common iliac artery. A 10-mm prosthetic conduit (Dacron or polytetrafluoroethylene [PTFE], DuPont, Wilmington, Delaware) will accommodate the largest needed sheath. The conduit should be tunneled under the inguinal ligament through a femoral counter incision. The distal end of the conduit is then clamped to the drapes and accessed in the usual fashion. After the EVAR is completed, most operators elect to anastamose the distal end of the conduit to the common femoral artery in an end-to-side fashion. Alternately, the conduit can be removed, leaving a small “stump” on the common iliac artery.
Imaging
Equipment
High-quality fluoroscopy and angiographic equipment is essential to successful EVAR. A contemporary fixed-imaging unit as part of a “hybrid” operating room is increasingly utilized in many institutions. The performance of fenestrated or branched EVAR mandates this level of imaging. Portable C-arm fluoroscopy with a modern unit is acceptable for standard EVAR and/or TEVAR but cannot provide the versatility and imaging capabilities of fixed units.
Gantry Positioning
The precision of proximal endograft deployment with EVAR and TEVAR is considerably enhanced by adjusting the gantry of the fluoroscope to be perpendicular to the axis of the aortic neck. A large percentage of infrarenal aortic necks have a modest amount of anterior angulation that should be adjusted for by adding an appropriate amount of cranial tilt to the fluoroscopy unit. The amount needed can be estimated by the preoperative CTA. The majority of infrarenal aortic necks have 5 to 15 degrees of cranial angulation, but this can be as much as 30 to 40 degrees. Adjusting the left and/or right obliquity is also helpful in precisely locating the origin of the lowest renal artery. The left renal artery typically comes off the aorta a bit posterior to the centerline, whereas the right renal artery most commonly originates somewhat anterior to the centerline. An appropriate amount of left anterior oblique (LAO) angulation will give the proper orientation to the renal arteries in most cases. Frequently, this translates to approximately 10 to 20 degrees of LAO. These adjustments are particularly important in patients with shorter and angulated aortic necks.
Gantry position is also critical in the performance of TEVAR. A significant LAO of 30 to 75 degrees is routinely needed to best visualize the aortic arch and the origin of the subclavian, carotid, and innominate vessels. If the distal extent of a thoracic endograft needs to be deployed close to the celiac vessel, a true lateral (or >75 degree obliquity) is required.
Endovascular Aortic Repair Deployment
Wire Placement
After bilateral femoral access is achieved (open and/or percutaneous), a floppy J-wire should be placed in the proximal thoracic aorta, and over a catheter, exchanged for a stiff guidewire (Lunderquist, Cook Medical; Amplatz Super Stiff, Meier, Boston Scientific; Natick, Mass). These very stiff guidewires should not be routinely advanced in a bare fashion and never without fluoroscopic visualization. Failure to routinely visualize the location of these stiff wires in the aortic arch increases the risk of inadvertent cannulation of an arch vessel, plaque disruption, and stroke. After the stiff wire is placed, the distal end of the wire should be marked on the operative drape so that a stable position is ensured throughout the procedure. Through the contralateral femoral artery, a pigtail catheter is then placed just above the renal arteries, which are usually between L1 and L2. If there is concern about iliac occlusive disease, an angiogram should be taken at the aortic bifurcation, and the occlusive disease should be treated as previously described. If there is uncertainty regarding the correct main body length to use, an aortagram with a 1-cm marker catheter can be performed to confirm the distance between the lowest renal artery and the ipsilateral hypogastric artery. Otherwise, no arteriography should be performed until the undeployed main component is placed in the pararenal area. Particularly in patients with significant iliac and/or aortic neck tortuosity, placement of the undeployed endograft in the pararenal aorta may alter the relative position of the renal arteries, making the preinsertion arteriography inaccurate.
Delivery of the Main Device
The contralateral gate or limb of the main device should be appropriately oriented by fluoroscopy before insertion (Fig. 90-4A). This orientation should be re-checked as the device is being advanced, and any rotational adjustments made while the device is also being axially moved. Attempts to rotate the device statically may not translate to the entire device, particularly in tortuous and/or small iliac arteries. Failure to follow these principals can cause a relative twist in the endograft.
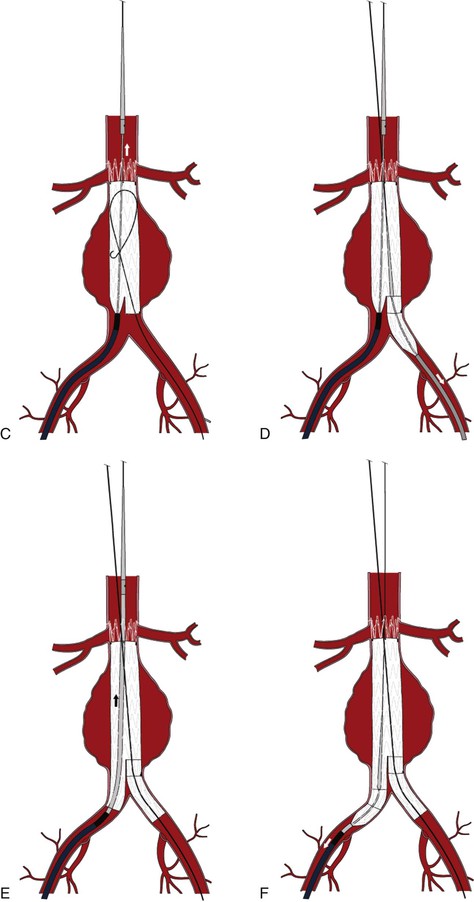
Figure 90-4 A, Placement of the undeployed main body in a position immediately below the lowest renal artery. Note the orientation of the contralateral gate. B, Deployment of the main body, stopping after the contralateral gate is opened. C, Deployment of the suprarenal bare stent. D, Deployment of the contralateral iliac limb after a retrograde arteriogram marking the location of the hypogastric artery. E, Placement of the ipsilateral iliac limb. F, Deployment of the ipsilateral iliac limb.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree