Chapter 89
Technique
Endovascular Therapeutic
Timothy M. Sullivan, Addi Z. Rizvi
Based on a chapter in the seventh edition by Makoto Sumi and Takao Ohki
The advent of endovascular therapy for the treatment of patients with arterial occlusive and aneurysmal disease has revolutionized the care of vascular patients. Minimally invasive endovascular techniques are typically performed under local anesthesia, are less stressful on patients compared with open surgical procedures, and provide for quicker return to function, especially in older, debilitated individuals. The tradeoff has typically been durability compared with open surgical options. This chapter reviews the currently available devices and techniques for endovascular therapy, their applications, limitations, and potential complications.
Balloon Angioplasty
It was a group of vascular surgeons, Fogarty et al,1 who first described an endovascular balloon catheter for extraction of arterial emboli and thrombi in 1963. Transluminal treatment of arterial stenosis using progressive arterial dilation was subsequently advanced by Dotter and Judkins in 1964.2 Eventually, Gruentzig and Hopff 3 developed the concept of utilizing a nonelastomeric balloon to dilate arterial lesions. Dilation of an arterial stenosis by balloon angioplasty results in disruption of the atheromatous luminal ring, focal luminal dissection, and stretching of the adventitia and media, with a resultant increase in overall luminal diameter. Currently, standalone balloon angioplasty or percutaneous transluminal angioplasty (PTA) has largely been supplanted by PTA with stenting. Some of the shortcomings of PTA (early restenosis, flow-limiting dissections) have been obviated with placement of an endovascular stent. However, the promise of drug-eluting balloons may bring back PTA to the forefront for the vascular interventionalist. Thus, a thorough understanding of PTA remains important.
Balloon Catheter Types
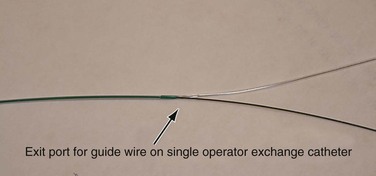
There are two types of balloon catheters in common use, over-the-wire (OTW) and single-operator exchange (SOE). In the OTW system, the guide wire enters at the distal end of the balloon and exits at the proximal end of the catheter. Common lengths for OTW balloon catheters are 80 cm and 130 to 150 cm. It is important to remember that OTW balloon catheters in the 130 to 150 cm lengths typically require a guide wire of 260 cm or greater if the lesion is distant from the arterial access site, to have enough wire length outside the patient to encompass the entire length of the balloon catheter. With the SOE system (commonly referred to as rapid exchange or monorail), the wire enters at the distal end of the balloon and exits the catheter along the shaft 8 to 10 inches proximal to the balloon (Fig. 89-1). The remainder of the catheter shaft is not supported by a guide wire. Advantages of the SOE system are that it can be loaded and advanced by a single operator who can stabilize (“pin”) the wire close to the insertion site, rather than requiring an assistant to pin the wire at the far end of an OTW balloon system. An exchange length guide wire is not necessary for SOE systems, which reduces the time needed for exchanges. Disadvantages of SOE systems include less pushability and trackability compared with a similar OTW system. SOE catheter balloons tend to be lower profile than their OTW counterparts and are more commonly used in the coronary and tibial vascular beds, where vessel diameters are much smaller.
Balloon Characteristics
Compliance
All PTA balloons are composed of plastic polymers. The characteristics of the polymer affects the balloon’s overall performance with regard to compliance, burst pressure, and puncture resistance. PTA balloons have a stated nominal pressure, which is the amount of pressure required (reported in atmospheres of pressure) to inflate the balloon to its stated diameter. Some PTA balloons will expand beyond their stated diameter as the atmospheric pressure is increased. The rated burst pressure is the reported pressure at which 99.9% of the balloons tested by the manufacturer will not burst. A PTA balloon’s compliance is the amount the balloon will expand beyond its determined diameter as inflation pressure is increased. Compliant balloons will tend to expand or stretch in the direction of least resistance. When such a balloon expands across a stenosis there is a reduction in the dilating force at the stenosis due to more dilation in the adjacent non-stenotic artery, resulting in more potential for injuring the artery adjacent to the treated area. Noncompliant balloons (typically made of polyethylene terephthalate or nylon reinforcement) have much less expansion in diameter or length because increased pressure is applied during inflation. Typically, they also have a higher rated burst pressure. Most interventionalists prefer noncompliant balloons because they provide a higher, uniform dilating force at the stated diameter and length, which is desirable when treating highly calcific lesions. Compliant balloons are typically constructed of a polyolefin copolymer or polyethylene. One advantage of a compliant balloon is that a single balloon can potentially treat vessels of varying diameter. Today, the most common role for a compliant balloon is for “sealing” thoracic and abdominal aortic stent grafts at the proximal and distal landing zones, within the aorta or graft overlap (Coda Balloon, Cook, Bloomington, Ind; Reliant Balloon, Medtronic, Santa Rosa, Calif; Q-50 Balloon, WL Gore, Flagstaff, Ariz).
Profile and Trackability
Profile is the overall diameter of the balloon and shaft. Smaller profile balloons allow passage across occlusions or severe stenoses. Balloons based on a 0.014-inch guide wire platform utilize a thinner plastic polymer and typically have a reduced diameter for the catheter compared with a 0.035-inch guide wire platform balloon.
Additional characteristics of balloons that describe their ability to be delivered to a remote lesion include trackability and pushability. Trackability is the overall ability of the balloon and the catheter to follow the course of the guide wire and to conform to tortuous anatomy. Features of the balloon and catheter that contribute to trackability include catheter lubricity and catheter flexibility. The pushability of a balloon catheter refers to the columnar force that is transmitted from the shaft of the balloon catheter to the tip of the balloon. Balloon catheter systems designed for a 0.035-inch guide wire have much higher pushability compared with 0.014-inch guide wire balloon catheter systems. Hydrophilic or lubricious coatings to the balloon and catheter also contribute to pushability. A uniform transition of the taper of the tip of the balloon with the wire also improves pushability of the balloon because it eliminates an abrupt leading edge on the balloon that typically impedes passage of the balloon through occlusions or severe stenosis.
Specialty Balloons
Cutting Balloon Angioplasty
A number of devices for PTA have been developed for specialized purposes, in an attempt to either improve the initial results of angioplasty or to reduce the incidence of restenosis. Cutting balloons are constructed with metal atherotomes (microsurgical blades) mounted longitudinally along the balloon surface, designed to create controlled “incisions” along the length of the lesion, with the intention of reducing the extent of vessel injury and the complexity of the arterial dissection created during conventional PTA.
Although the majority of experience with cutting balloon angioplasty (CBA) has been gained in the coronary circulation, its use has gained popularity in treating early, hyperplastic vein graft stenoses. Schneider et al4 reported 164 stenoses in 124 vein grafts treated over a 12-year period. Forty-two were treated with open surgery, 57 with PTA, and 62 with CBA. The stenosis-free patency at 4 years for open surgery, CBA, and PTA were 74%, 62%, and 34% respectively. Pseudoaneurysms developed in two patients who underwent CBA, one of whom required surgical repair. The authors concluded that CBA is a reasonable initial treatment option for infrainguinal vein graft stenosis in most patients, with superior results to PTA and comparable to open surgery. Other authors have reported technical success with CBA in infrainguinal vein grafts, but note that CBA may be associated with a significant risk of complications, principally perforation. Garvin et al5 performed 109 CBA procedures on 70 bypasses, with a complication rate of 11%.
Finally, CBA has been used to treat hyperplastic stenoses in native hemodialysis fistulas. Wu et al6 compared their experience with CBA and standard angioplasty with high-pressure balloons in the treatment of resistant native hemodialysis fistulas. Initial technical success was similar, but long-term patency was statistically superior in the CBA group. Of note, although there were no device-related complications in the CBA group, 6 of 35 patients in the high-pressure balloon group had extravasation of contrast after the procedure.
Cryoplasty
Cryoplasty (endovascular cryotherapy) has been investigated to improve the results of PTA in the arterial system, and to potentially avoid the use of stents. Animal studies have suggested that cryotherapy alters the healing response after angioplasty, principally by inducing smooth muscle cell apoptosis rather than necrosis, theoretically reducing the incidence of myointimal hyperplasia.7 The balloon catheter is inflated with nitrous oxide during the procedure, delivering cold thermal energy to the arterial wall during balloon inflation. Early experience with the technique from a multicenter registry was reported by Laird et al.8 One hundred two patients with short (<10 cm) femoropopliteal lesions were treated with cryoplasty at 16 centers. Initial technical success was achieved in 85%. Primary patency by duplex ultrasound was 70% at 9 months.
Longer term results from the technique have been variable. The Cryoplasty CLIMB Registry9 reported on 100 patients with critical limb ischemia (CLI) treated for infrapopliteal stenoses or occlusions, with a mean lesion length of 55-mm. Initial technical success was 95%; stents were required in 17%. Primary patency at 12 months was 56%. The authors concluded that the technique was effective, and that results were in the range of those achieved with conventional PTA. The use of cryoplasty for lesions less than 50 mm was not recommended. The BTK Chill Trial10 analyzed the results of primary cryoplasty in the infrapopliteal arterial segment in patients with CLI. In 108 treated limbs, technical success was achieved in 97%, with stent placement required in only 3 patients (2.7%). At 1 year, major amputation was avoided in 85% of patients. Target lesion revascularization (TLR) was required in 21% at 1 year. The authors concluded that the technique was safe and effective, and recommended cryoplasty as a primary treatment option in patients with CLI secondary to infrapopliteal disease.
Other investigators have reported less favorable results with the technique. Samson et al11 evaluated their results using cryoplasty in 92 lesions in 64 consecutive patients with lesions of the superficial femoral artery. Initial technical success was achieved in 88%, and stent placement was required in 10%. Freedom from restenosis was 57% and 49% at 12 and 24 months, respectively. At their institution, cryoplasty added $1700 to the cost of each procedure. As a result of these data, these authors no longer use the technique in their practice. Schmieder et al12 reviewed their results in 71 patients treated for both de novo and restenotic complex arterial lesions (multifocal, diffuse, or occlusions) in the lower extremities. One-year primary patency was 17% for patients with lesions of the native arteries and 28% for patients treated for in-stent restenosis. The authors reported a similar increase in cost compared with conventional PTA. The COLD study13 randomized 86 patients with popliteal artery lesions to cryoplasty or conventional PTA. On an intent-to-treat basis, the initial technical success was 35% for cryoplasty and 54% for conventional angioplasty (P = .02), with stents required in 30% of cryoplasty patients and in 39% of those who underwent conventional PTA (P = .34). Patency at 9 months was 79% for cryoplasty and 67% for PTA (P = .14). Although there has been enthusiasm for crypolasty, it remains unclear as to its ultimate role in the treatment of patients with peripheral artery disease, especially considering the additional cost of the procedure compared with conventional therapy.
Drug-Coated Balloons
Although many physicians have embraced an “endovascular first” policy when treating infrainguinal arterial disease, restenosis following percutaneous intervention remains a significant issue, especially for complex lesions. In addition, intravascular stents may induce myointimal hyperplasia and subsequent restenosis. Antiproliferative agents have been used as drug-eluting stents (DESs) in the coronary circulation with excellent results. The Cypher stent (Cordis, Warren, NJ), first used in 1999, was coated with sirolimus, an immunosuppressive drug that limits myointimal hyperplasia principally through inhibition of smooth muscle cell proliferation.14 Paclitaxel, an antineoplastic drug that also reduces myointimal hyperplasia by decreasing smooth muscle cell proliferation and migration has also been utilized successfully in the coronary circulation as the TAXUS stent (Boston Scientific, Natick, Mass). Unfortunately, these results have not yet been achieved in the infrainguinal arterial bed. The “ideal” intervention for infrainguinal arteries would be minimally invasive, not leave a metal stent in the hostile environment of the superficial femoral artery, and provide long-term durability; these qualities could be achieved by using antiproliferative drug delivery via balloon catheter, without the use of metal stents. Experimental studies suggest that paclitaxel-coated balloons are able to deliver substantial amounts of the drug to the arterial wall during short (1 minute) periods of balloon inflation, and that inhibition of smooth muscle cell proliferation is achieved over time.15
Clinical trials of drug-coated balloons (DCBs) are currently underway in the United States, and the results of several studies from Europe are available for review. The THUNDER trial17 randomized 154 German patients with stenotic lesions (mean lesion length 7.4 cm) of the femoral-popliteal segment into three groups: (1) paclitaxel-coated balloon with standard nonionic contrast; (2) standard angioplasty balloon with paclitaxel added to the contrast; and (3) standard angioplasty balloon with standard nonionic contrast (control group). Stents were utilized selectively, that is, only for significant residual stenosis following angioplasty, which was required in more patients in the control group compared with the paclitaxel-coated balloon group (22% versus 4%; P = .009). At 6 months, all patients underwent angiography in a blinded fashion. Patients in the paclitaxel-coated balloon group had significantly lower late lumen loss and restenosis (17% versus 44%; P = .001), and lower rates of TLR through 24-month follow-up (7% versus 52%). Group 2 (paclitaxel-added contrast) did not show improvement over the control group. Although patients in this trial were treated for stenosis rather than occlusion, the results were very encouraging. The FemPac trial17 was another multicenter German study that evaluated paclitaxel DCBs in the femoral-popliteal segment, randomizing 87 patients to either standard PTA or DCBs. At 6 months angiographic follow-up, the DCB group had significantly less restenosis (19% versus 47%; P = .045), and at 18 to 24 months follow-up, a lower rate of TLR (3% versus 33%; P = .002). Like the THUNDER trial, no adverse events were associated with the use of DCBs.
The DEBELLUM trial18 randomized 50 patients with 122 atherosclerotic lesions that had more complex disease than in previously reported studies (96 stenoses, 26 occlusions) in the femoral-popliteal (75%) and infrapopliteal (25%) arteries. Patients who required stent placement for residual stenosis or dissection following angioplasty were excluded from the trial. Restenosis at 6 months was 9% for the DCB group compared with 29% in the standard PTA group (P = .03).
Favorable preliminary results of several other DCB trials have been presented, but have not yet been subjected to peer review. Results from these early trials suggest that for many patients with femoral-popliteal stenosis, DCB angioplasty may provide durable results compared with standard PTA; further investigation will be required to determine if the therapy is viable for more complex lesions and occlusions and in those patients who require stent placement for angioplasty failure.
Technique
Approach to the Lesion
Whether it is the renal, mesenteric, iliac, femoropopliteal, or tibial vessels, preprocedural preparation is important when determining the access site. Often, imaging (duplex ultrasound, computed tomographic angiography [CTA], or magnetic resonance angiography [MRA]) of the intended lesion to be treated is available before intervention. This will assist the interventionalist in determining the appropriate access site. Confounding patient factors may also determine the access vessel. Examples include patients with previous common femoral artery open surgery where scarring or potential injury to a previous bypass should be avoided if possible, or a previous aortic stent graft, which makes retrograde access to the contralateral leg difficult. Without such factors, the shortest distance from the access vessel to the target lesion is typically desired because this provides the most control and longitudinal support with catheters and wires. Most interventionalists are right-hand dominant, and the retrograde right common femoral access approach is typically used. However, for treating distal tibial occlusive disease, an ipsilateral antegrade common femoral approach may be more desirable. The retrograde brachial approach is often optimal when treating lesions involving the celiac or superior mesenteric artery, iliac occlusive disease is not accessible from the femoral approach, or for treating brachiocephalic disease.
Once arterial access is obtained, a hemostatic sheath is placed. Currently, the majority of wires, catheters, balloons, and stents for peripheral interventions can be performed through a 6F or smaller sheath. Arteriography is performed to identify the lesion and the severity. Various techniques are available to mark the lesion. Often bony landmarks, roadmapping, or even marking the lesion on the screen with an erasable marker can be used to “mark” the lesion. The use of a Glow n’ Tell (LaMaitre Vascular, Burlington, Mass) radiopaque marking tape, particularly for femoral and tibial interventions is simple and useful. Once the decision is made to treat the lesion, the patient should be systemically heparinized.
Lesion Crossing Techniques.
There are various techniques for crossing an atherosclerotic lesion, and these vary based on the length of the lesion and the degree of stenosis or occlusion. Basic principles are to have either a support catheter or the proximal end of the sheath as close to the lesion as possible to provide the most support for working the wire and catheter. For highly stenotic lesions, a 0.014- or 0.018-inch system may be desirable. Wires with hydrophilic tips tend to cross these types of lesions easier, but caution must be taken because they are also easier to pass into the subintimal plane. The Quick-Cross (Spectranetics, Colorado Springs, Colo) catheter (available in 0.014-, 0.018-, and 0.035-inch sizes) is a useful tool for traversing either complex stenosis or occlusions. When using the appropriate wire, the catheter provides optimal support, is hydrophilic, and has virtually no transition between the wire and the catheter tip. Once the lesion is crossed, the contrast injection through the catheter is performed to ensure that the wire is intraluminal. At this point, usually a stiffer support wire can be exchanged over a catheter to provide more support for the intervention.
Balloon Catheter Selection, Placement, and Inflation
Balloon Selection.
A balloon diameter that is slightly oversized to the vessel diameter is chosen. A more conservative balloon diameter may be appropriate in situations in which avoidance of adjunctive stenting is desired. Further, in severely calcified lesions, a more conservative dilation may be used to avoid artery rupture. Table 89-1 lists typical balloon diameters for intended vessels to be treated.
Table 89-1
Typical Balloon Size for Specific Arterial Vessels
| Lesion Location (artery) | Balloon Diameter (mm) | Balloon Length (mm) |
| Internal carotid | 4-6 | 2 |
| Common carotid | 6-8 | 2-4 |
| Vertebral | 3-5 | 2 |
| Subclavian | 6-8 | 2-4 |
| Axillary | 5-7 | 2-4 |
| Aorta | 10-20 | 2-4 |
| Renal | 5-7 | 2-3 |
| Common iliac | 6-10 | 2-4 |
| External iliac | 6-8 | 2-8 |
| Superficial femoral | 5-7 | 2-150 |
| Popliteal | 4-6 | 2-4 |
| Tibial | 2-3 | 2-100 |
Ideally, a balloon length just slightly longer than the lesion length is chosen. Current balloon lengths of up to 220 mm are available and are ideal for treating long segment femoropopliteal or tibial lesions with a single inflation. Techniques to determine the optimal balloon diameter include starting with a smaller diameter balloon and upsizing as appropriate, using digital subtraction software to determine the lesion length and diameter, and finally, utilizing an intravascular ultrasound (IVUS) catheter to determine vessel diameter. If the patient has CTA before the procedure, the vessel diameter can be determined from this. Most balloon catheters come in 80 cm or 130 to 150 cm shaft length. If treating from the common femoral approach, carotid and brachiocephalic interventions and contralateral popliteal and tibial interventions require the longer shaft. The shorter 80-cm shaft can be employed for mesenteric, renal, iliac, and proximal contralateral superficial femoral interventions when approached from the common femoral access. The balloon is prepped by aspirating air from the balloon port and replacing it with a 25% to 50% strength contrast to provide for more consistent balloon inflation.
Balloon Placement.
Additional contrast injection through the sheath or guide catheter can be performed to correctly mark the lesion. On occasion, it may be difficult to pass the balloon catheter across the stenotic lesion. This may require exchanging out for a stiffer wire, advancing the sheath or guide catheter closer to the lesion, or switching to either a lower profile balloon or 0.014-inch based system for predilation if necessary, before placing the larger balloon.
Balloon Inflation.
Once appropriate positioning of the balloon is confirmed, inflation of the balloon is performed using dilute contrast (50:50 contrast and saline) using an inflation device. Current balloons inflate at the middle initially and at the proximal and distal ends lastly. It is important to watch inflation of the balloon in real time to see if the lesion is appropriately treated. Typically, the balloon is inflated up to the rated nominal pressure; inflation times vary based on operator preference. The balloon is completely deflated and withdrawn out of the sheath, and contrast injection through the sheath is performed to assess the PTA result. It is important to maintain guide wire access across the treated lesion in case additional intervention is necessary. If treating an iliac lesion from a distal retrograde femoral access site, the guide wire can be exchanged out for a flush catheter placed in the aorta or more proximally to perform the final angiogram. If the completion angiogram shows a residual dissection or stenosis, reinflation of the balloon across the lesion can be performed. Other adjuncts to determine success of treatment include imaging the lesion in different oblique views, assessing if there is a pressure gradient across the treated area, or interrogating the treated lesion with intravascular ultrasonography.
Treating Chronic Total Occlusions
Subintimal Angioplasty
The technique of subintimal angioplasty was first described by Bolia et al44 in 1989 to treat chronic total occlusions. The concept entails creating an intentional subintimal dissection plane with a guide wire. The guide wire is typically hydrophilic in nature and is advanced with the tip of the wire formed in a gentle loop. The looped tip is then advanced together with a hydrophilic support catheter along the subintimal plane across the occlusion, and then the wire and catheter are redirected back into the true lumen distal to the occlusion. Contrast injection through the catheter confirms reentry back into the true lumen. A stiffer wire may be exchanged, and balloon angioplasty of the occlusion commences. It may be necessary to sequentially dilate a tract starting with small diameter or lower profile balloons and eventually work up to the intended balloon diameter. Subintimal angioplasty typically has been utilized to treat iliac and femoropopliteal occlusions. This technique has been successful in highly calcified lesions, long (>15 cm) occlusions, and “flush” occlusions of the superficial femoral artery. Reported 12-month patency rates are typically <70%.45 Unfortunately, the technical failure rate of subintimal angioplasty is up to 26%.46 The main reason for failure of subintimal angioplasty is the inability to reenter the true lumen distal to the occlusion. To reduce this failure rate, a number of devices are available for current use, as described in the next section.
To increase the technical success rate of subintimal angioplasty, there are devices specifically designed to assist in reentering the true lumen once guide wire placement across a chronic total occlusion (CTO) in the subintimal plane has been performed. Another strategy to treat CTOs involves the use of specific devices in an effort to stay intraluminal while traversing the occluded segment (as opposed to the subintimal space for reentry devices).
Reentry Devices
Outback LTD
The Outback LTD (Cordis Corporation, Bridgewater, NJ) is a device designed to reenter the true lumen once an occlusion has been crossed in the subintimal plane. The device has a single 0.014-inch compatible lumen that works through a 6F sheath. Once the occlusion is crossed and reentry into the true lumen is desired, a 22-gauge nitinol curved needle is oriented in the direction of the true lumen and deployed. Orientation is determined by the radiopaque marks on the tip of the catheter “L” and “T.” The “L” marker is orientated toward the true lumen and the C-arm/fluoroscope is angled 90-degrees orthogonally, such that the “T” marker appears. Once the needle is in the true lumen, the 0.014-inch guide wire is advanced into the true lumen, and intervention on the lesion can be performed.
Pioneer
The Pioneer (Volcano Corporation, San Diego, California) is also a true reentry catheter that utilizes IVUS to assist in directing the reentry needle into the true lumen. The device is advanced up the subintimal plane just beyond the occlusion near the true lumen. The catheter is attached to an IVUS console. In real time using color Doppler, the true lumen is oriented in the direction of the 24-gauge reentry needle, and the needle is deployed. A 0.014-inch guide wire is then passed into the true lumen, and once the device is removed, intervention of the occlusion is performed.
Enteer
The Enteer Re-entry System (Covidien/eV3, Plymouth, Minn) is a balloon catheter-based reentry device. It is designed to be used once the interventionalist has gained access across the lesion in the subintimal plane. It is a flat balloon that has two separate 180-degree opposing radiopaque exit ports on the balloon. The balloon is inflated in the subintimal space, and a special directable and/or steerable wire is used to exit the appropriate luminal-facing exit port to reenter the true lumen. The benefits of this device are the relatively low cost and no need for significant capital investment compared with other reentry devices.
Device Selection
The benefits of the Outback LTD and Pioneer reentry catheter are that both have a directable nitinol needle that may puncture through the plaque overlying the subintimal plane to gain reentry back into the true lumen. The Pioneer catheter is particularly useful for treating aortoiliac occlusions at the aortic bifurcation. Entry back into the aortic lumen once the iliac occlusion has been traversed along the subintimal plane can be carefully visualized in real time, utilizing both IVUS and fluoroscopy. The Enteer reentry balloon lacks this and relies on a steerable wire to gain reentry into the true lumen. The downside of the Pioneer catheter is the up front investment in an IVUS console for its use.
Intraluminal Devices
Crosser
The Crosser (Bard Peripheral Vascular, Tempe, Ariz) device consists of a vibration generator that is attached to a special stainless steel–tipped catheter. This results in the tip of the catheter vibrating at a low amplitude with a high frequency (20 kHz). Once activated, the tip of the catheter can break through atheromatous occlusions. The device for peripheral applications works through a 7F sheath over a 0.014-inch wire. The catheter is advanced across the occlusion with the guide wire within the catheter, and once it is felt that the lesion has been crossed, the guide wire is advanced into the true lumen beyond the occlusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree