Chapter 40
Systemic Complications
Respiratory
Leigh Ann Slater, Pamela A. Lipsett
Based on a chapter in the seventh edition by Jayme E. Locke and Pamela A. Lipsett
A major contributor to perioperative morbidity and mortality is postoperative pulmonary complications.1–5 The incidence ranges from 5% to 80%, depending on the population studied and the criteria used to define complications.2,5 Comprehensively defined, atelectasis, bronchospasm, bronchitis, pneumonia, aspiration pneumonitis and pneumonia, exacerbation of chronic obstructive pulmonary disease (COPD), pulmonary edema, and various forms of upper airway obstruction are considered postoperative pulmonary complications2 (Box 40-1). This chapter discusses identification and prevention of pulmonary complications with particular emphasis on amelioration of modifiable risk factors and newer treatments of respiratory failure.
Risk Factors
Preoperative risk factors for pulmonary complications include both patient-related and procedure-related risks (Table 40-1).7–12 Procedure-related risk factors may be more important, but except in the choice of procedure, they may not be as readily modifiable. Perioperative and postoperative risk factors include those associated with aspiration, poor inspiratory effort, and overfeeding. Many of these risk factors are modifiable.
Table 40-1
Potential Patient-Related and Procedure-Related Risk Factors for the Development of Postoperative Pulmonary Complications
| Patient-Related Risk Factors | Procedure-Related Risk Factors |
| Chronic obstructive lung disease | Surgical site |
| Asthma | Duration of surgery (>3 hours) |
| Tobacco use | Type of anesthesia |
| Health status (American Society of Anesthesiologists class >2) | Endotracheal intubation |
| Obesity | Long acting nondepolarizing blockade |
| Age | Large tidal volumes (10-12 mL/kg) |
| Upper respiratory infection | High FIO2 |
| Metabolic factors | |
| Heart failure | |
| Poor exercise tolerance | |
| Functional dependence | |
| Abnormal lung scan |
Patient-Related Risk Factors
Chronic Lung Disease
Preexisting lung disease is the most important patient-related risk factor for postoperative pulmonary complications. Reported unadjusted relative risks for patients with underlying pulmonary disease range from 2.7 to 6.6. Preoperative findings consistent with COPD include decreased breath sounds, prolonged expiratory phase, rales, wheezes, rhonchi, and chest radiographic evidence of hyperinflation. Studies demonstrate postoperative pulmonary complications in up to 26% of patients with these findings versus only 8% of patients without these findings. Nevertheless, there is no identified lower threshold of pulmonary function below which surgery is absolutely contraindicated.13–20 Even extremely high risk patients may proceed to surgery if the indication is sufficiently compelling and the patient and surgeon are willing to assume the added risks.
Asthma
Despite early reports of higher than expected rates of postoperative pulmonary complications, it has since been shown that patients with well-controlled asthma and peak flow measurements better than 80% of predicted can proceed to surgery at average risk.6,14–15,17 Patients who have active use of asthma medications and emergency department or office visits within 30 days before anticipated surgery are at increased risk of postoperative pulmonary complications and should have their surgery delayed to permit adequate control of asthma before proceeding.
Smoking
Current smoking increases the risk of postoperative pulmonary complications threefold (see Chapter 27 for further discussion).1,2,21–24 Whereas textbooks and older literature suggested that recent discontinuation of smoking increased risk of postoperative pulmonary complications purportedly through increased sputum production, a recent systematic review found nine studies examining the effect of timing of discontinuation of smoking on perioperative complications. One of the studies demonstrated a beneficial effect of recent quitting compared with continued smoking. Meta-analysis demonstrated that quitting smoking within 8 weeks before surgery was not associated with either an increase or a decrease in postoperative pulmonary complications for four studies examining this specific group of complications (relative risk, 1.18; 95% CI, 0.95-1.46).22,24 Thus patients should be strongly encouraged to stop smoking whenever they present for medical care.
General Health Status
The commonly used American Society of Anesthesiologists classification considers any systemic disease that affects activity or is a threat to life, including chronic lung disease, and it generally correlates well with pulmonary risk. An American Society of Anesthesiologists class higher than 2 confers a 1.5-fold to 3.2-fold increased risk of postoperative pulmonary complications.2
Obesity
Physiologic changes in morbid obesity include reduced lung volumes (functional residual capacity [FRC]), ventilation-perfusion mismatch, and relative hypoxemia.25,26 As similar changes are seen under anesthesia, the specific contribution of obesity to the incidence of postoperative pulmonary complications is difficult to quantify. Calligaro et al studied 128 aortic surgery patients, 26 of whom were obese, and demonstrated a pulmonary complication rate of 27% in obese patients versus 17% in nonobese patients, with an unadjusted risk ratio of 1.6.27 A careful analysis would suggest that patients with both comorbidities and obesity are at increased risk, and identification and treatment of these associated diseases is important for preoperative optimization to minimize complications.
Age
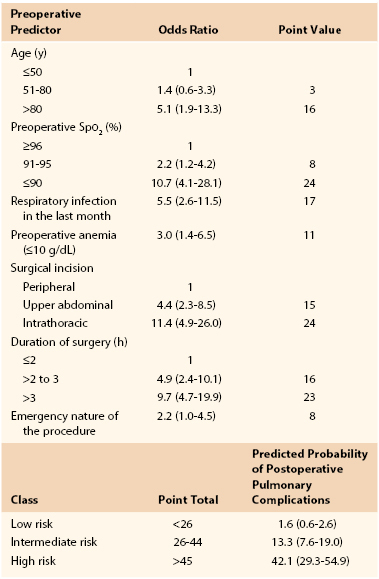
Early studies suggested an increased risk of pulmonary complications with advanced age,28 but they were not adjusted for overall health status or preexisting pulmonary disease. Canet’s straightforward risk model9 for estimating risk of postoperative pulmonary complications assigns 3 risk score points for age of 51 to 80 years and 16 points for age older than 80 years. Overall, low risk is assigned for less than 26 points (rate of 1.6), intermediate risk for 26 to 44 points (rate of 13.2), and high risk for more than 45 points (rate of 42.1) (Table 40-2).9
Upper Respiratory Infection
Patients who have had a respiratory infection within the month before surgery are at increased risk of postoperative pulmonary complications. In a study of 1624 patients, the odds ratio was 5.5 (2.6-11.5) if a recent infection had occurred. It would seem wise to avoid elective surgery in this setting.2,10
Metabolic Factors
A multifactorial risk index for postoperative respiratory failure identified two metabolic risk factors.29 Albumin level of less than 3 g/dL and blood urea nitrogen concentration of more than 30 mg/dL each predicted increased risk with odds ratios of 2.53 and 2.29, respectively. Etz et al30 examined outcomes after thoracoabdominal aneurysm repair and also found an association between preoperative renal insufficiency (blood urea nitrogen concentration >24 mg/dL) and the development of pulmonary complications, with incidence of 42% versus 25% (P = .03).
Procedure-Related Risk Factors
Table 40-1 summarizes procedure-related risk factors for perioperative pulmonary risk. These include operative site, duration and method of anesthesia, and choice of neuromuscular blocker.2,10
Surgical Site
Surgical site is the most important procedural risk factor for postoperative pulmonary complications, with the incidence of complications inversely related to distance of the incision from the diaphragm. Choice of incision may adversely influence respiratory muscle and diaphragmatic function.6,31,32 Complication rates for thoracic surgery range from 19% to 59%; for upper abdominal surgery, from 17% to 76%; and for lower abdominal surgery, from 0% to 5%.30–33
Duration of Surgery
Operative duration of more than 2 to 3 hours confers a higher risk of postoperative pulmonary complications.6,10,33 A study of 520 patients demonstrated such complications in 8% of surgeries lasting less than 2 hours versus 40% of procedures lasting more than 4 hours. When one is available, it is prudent to pursue a less ambitious, briefer procedure in the high-risk patient.
Type of Anesthesia
Whereas the data are conflicting and generally of low quality, two large meta-analyses as well as retrospective and randomized trials suggest that postoperative pulmonary complication rates are lower for patients who have spinal or epidural anesthesia for surgery or epidural analgesia postoperatively.34–39 In addition, a meta-analysis by Block et al34 demonstrated that both static and dynamic postoperative pain relief is improved with epidural analgesia. A classic clinical trial performed at our institution by Norris et al39 randomized 168 patients undergoing abdominal aortic surgery to receive either thoracic epidural anesthesia with light general anesthesia or full general anesthesia alone with either intravenous or epidural patient-controlled analgesia postoperatively. Epidural patient-controlled analgesia correlated with a significantly shorter time to extubation. All treatment groups had otherwise comparable results, however, including time to intensive care unit (ICU) discharge, ward admission, first bowel sounds, first flatus, tolerance of clear liquids and regular diet, independent ambulation, and postoperative pain scores.
Type of Neuromuscular Blockade
The choice of neuromuscular blockade has a significant influence on postoperative pulmonary complications (see Box 40-1).1,2,15 In one prospective nonrandomized study, the difference in postoperative pneumonia between pancuronium and atracurium was 13% versus 5%.33 Evidence suggests that use of short-acting or intermediate-acting neuromuscular blockers is preferred. A prospective randomized study showed that patients treated with longer acting neuromuscular blockers were four times as likely to develop postoperative pulmonary complications.40
Perioperative and Postoperative Risk Factors
Perioperative and postoperative risk factors for postoperative pulmonary complications include those associated with aspiration, poor inspiratory effort, prolonged intubation, and overfeeding. Many of these risk factors are within our power to control and to remedy. Factors associated with aspiration and pneumonia include insufficient elevation of the head of the bed, immobility, overaggressive use of restraints, presence of nasogastric or other tubes that stent open both upper and lower esophageal sphincters, and oversedation or other toxic or metabolic causes of altered level of consciousness.41,42 In patients who must remain intubated, longer duration is associated with higher complication rates, and every effort should be made to extubate expeditiously, including protocols for daily weaning of sedation and trials of spontaneous respiration and readiness for extubation. Subglottic suctioning endotracheal tubes minimize risk of aspiration of microorganisms around the cuff. Patients who have been intubated for longer than 3 days should be properly assessed after extubation and before feeding with the expertise of speech-language pathologists to assess swallow function and supplemental cine-esophagography when bedside evaluation is equivocal. Attention should be paid to high gastric residuals and other signs of postoperative ileus or bowel obstruction. When gastric emptying is in question, a jejunal feeding access would be more appropriate.
Poor inspiratory effort is also multifactorial and may be associated with location of incision, poor pain control, suboptimal patient positioning and immobility, placement of chest tubes or braces or binders that impede full chest wall excursion, oversedation, neuromuscular defects, and poor nutrition. Each of these factors can be improved with appropriate attention to detail.
Conversely, overfeeding produces a surfeit of carbon dioxide that may be beyond the patient’s capacity to effectively exhale and can be demonstrated by a respiratory quotient greater than 1. Hypophosphatemia limits the patient’s available energy (adenosine triphosphate) for muscle contractility. Other electrolyte deficiencies, including hypokalemia, hypocalcemia, and hypomagnesemia, may also have an adverse impact. All may contribute to failure to be weaned from the ventilator and should be aggressively sought and corrected when present.
Preoperative Evaluation
A complete history and physical examination form the basis of preoperative risk assessment. Significant risk factors should be identified. Evidence suggesting underlying chronic lung disease should be sought, including a history of exercise intolerance, unexplained dyspnea, and cough. Physical examination should seek evidence of obstructive lung disease, such as decreased breath sounds, wheezes, rhonchi, or prolonged expiratory phase.14,15 Adjunctive studies including pulmonary function tests, arterial blood gas measurements, and chest radiography may be warranted but are no substitute for appropriate clinical evaluation and should not be done routinely.43,44
Consensus guidelines for preoperative practice emphasize general health and fitness and state that patients who demonstrate more than four metabolic equivalents (climbing two flights of stairs or running a short distance) are considered safe to proceed to surgery from a cardiac standpoint. However, patients with vascular disease are often unable to undergo exercise testing because of claudication or other symptoms. Cardiopulmonary exercise testing has been suggested as an objective measure of functional reserve that can be performed safely in such high-risk populations.45,46 Cardiopulmonary exercise testing is a ramped exercise test during which the electrocardiogram, blood pressure, and oxygen saturation are monitored and respiratory variables, including oxygen uptake and carbon dioxide excretion, are measured. A number of parameters can be derived, including the peak oxygen uptake (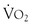 -peak), which is effort dependent, and oxygen uptake at the anaerobic threshold, which is effort independent. Whereas this test may be of future value in assessing perioperative risk, a review by Young et al44 suggests that currently there is insufficient evidence to justify its routine use in vascular surgery patients.
-peak), which is effort dependent, and oxygen uptake at the anaerobic threshold, which is effort independent. Whereas this test may be of future value in assessing perioperative risk, a review by Young et al44 suggests that currently there is insufficient evidence to justify its routine use in vascular surgery patients.
Pulmonary Function Testing
Except in pulmonary resection, there is considerable debate about the role of preoperative pulmonary function tests (PFTs) for perioperative risk stratification.2,43,47–50 Two reasonable goals justify preoperative PFTs in nonthoracic surgery: (1) identification of patients for whom the risk of the proposed open vascular surgical procedure outweighs the benefit or in whom a percutaneous or endoluminal approach would be better tolerated; and (2) identification of patients at higher risk, for whom more aggressive perioperative management is warranted. PFTs should not be obtained routinely for patients having abdominal surgery and should not be used as the primary reason for denying a patient an operation. PFTs might be useful for patients with COPD or asthma if clinical evaluation cannot determine whether the patient is at baseline with optimal reduction of airflow obstruction. Similarly, in patients with dyspnea or exercise intolerance that remains unexplained after clinical evaluation, the differential diagnosis may include cardiac disease or deconditioning, and the results of PFTs may change preoperative management and suggest a more aggressive treatment approach, such as perioperative corticosteroids, to achieve the best possible baseline functional level.50
PFTs provide an estimate of forced expiratory volume (FEV1) and forced vital capacity (FVC). An FEV1 or FVC of less than 70% and an FEV1/FVC ratio of less than 65% of predicted are associated with an increased risk of postoperative pulmonary complications.50 It again must be emphasized that most patients identified by PFTs as high risk for postoperative pulmonary complications can be identified equally well by astute history and physical examination.15,50
Arterial Blood Gas Analysis and Chest Radiography
No data suggest that hypercapnia identifies high-risk patients beyond those who would already be identified on the basis of established clinical criteria. In addition, abnormalities on chest radiographs are seen with increasing frequency with age, with uncertain significance. Chest radiographs also add little to the clinical evaluation of otherwise healthy patients, as was demonstrated in a meta-analysis of studies of routine preoperative chest radiography.51 Of 14,390 preoperative radiographs, there were only 140 unexpected abnormalities and only 14 cases in which the chest radiographic finding was abnormal and influenced management. The available literature does not allow an evidence-based determination of which patients would benefit from preoperative chest radiography. Chest radiography should, however, be performed if there has been a recent significant change in cardiac or pulmonary status.
Pulmonary Risk Indices
Cardiac risk indices have been used widely since 1977 to stratify the risk of perioperative cardiac complications.52 Several studies have attempted to formulate analogous pulmonary risk indices, but each study has significant limitations, often due to the complexity of the proposed index.53–57 The most simple and easily applied risk index is that proposed by Canet et al.9 In a study of 2464 patients, 252 postoperative pulmonary complication events were identified in 123 patients (5%), with higher 30-day mortality in those who suffered a postoperative pulmonary complication (19.5% [95% CI, 12.5%-26.5%] vs 0.5% [95% CI, 0.2%-0.8%]).9 The seven identified risk factors are listed in Table 40-2, with an area under the receiver operating curve of 88% (95% CI, 84%-93%). Aside from delaying or changing the magnitude of the planned procedure, most of the risk factors are not modifiable preoperatively. Whether more aggressive treatment of underlying pulmonary disease could improve oxygen saturation and therefore decrease risk is unproven. As a result, this index is more valuable to predict risk and to determine candidacy for surgery than as a tool for risk reduction strategies.
Perioperative Pulmonary Physiology
In a normal unstressed individual, respiratory rate is 10 to 12 breaths/min with a tidal volume of approximately 500 mL and a total minute ventilation of 5 to 6 L/min. Total minute ventilation consists of alveolar ventilation and the anatomic dead space, which is the portion of a breath not available for gas exchange. Anatomic dead space is roughly 130 to 180 mL per breath for a normal-size adult. Gas exchange depends on alveolar ventilation rather than on total minute ventilation.
Reduction in lung volumes resulting from anesthesia and surgery contributes to the development of atelectasis and other postoperative pulmonary complications.32,58,59 Patients typically experience a significant postoperative decrement in pulmonary function, especially after upper abdominal and thoracic incisions.31 The mechanisms causing the reduced lung volumes include decreased phrenic nerve activity and resultant decreased diaphragmatic function along with increased intercostal and abdominal muscle tone in response to postoperative pain and spinal reflex arcs. The most important physiologic change observed is a decreased FRC, the volume of air left in the lungs after normal expiration. Without intervention, FRC reaches a nadir at 24 to 48 hours postoperatively and returns to normal in uncomplicated low- to moderate-risk patients in approximately 1 week.
The relationship between FRC and closing capacity, the volume left in the lungs as the midsize and small airways begin to collapse, is a major determinant of postoperative pulmonary function and the resultant pulmonary morbidity.59,60 Normally, FRC is well above closing capacity, and tidal volumes result in air distribution throughout all alveoli. In the postoperative period, although FRC decreases dramatically, closing capacity remains unchanged. Patients in severe pain often cannot generate sufficient tidal volumes to overcome the closing capacity, resulting in lack of airflow to most alveoli with consequent atelectasis. Similarly, patients with mild pain may only be able to overcome closing capacity sufficiently to oxygenate alveolar units when at peak inspiration. This leads to ventilation-perfusion mismatch and consequent postoperative hypoxemia. Adequate pain control is essential to enable patients to breathe more deeply (increased tidal volume and FRC) and to cough more effectively (increased FEV1). Lower abdominal surgery is associated with similar changes but to a lesser degree, and reductions in lung volumes are not seen with extremity surgery.
Oxygen delivery is equal to the oxygen content of arterial blood multiplied by the cardiac output. The oxygen content of arterial blood is determined by the concentration of oxygen-saturated hemoglobin and dissolved oxygen in the blood (Table 40-3). Thus, it is evident that oxygen kinetics depend heavily on ventilation and perfusion. Oxygen is consumed peripherally at a rate of 200 mL/min in the average adult. Many factors affect oxygen consumption, including catecholamines and thyroid hormones. Oxygen delivery can be increased by improving oxygenation, correcting anemia, and optimizing cardiac output. Although optimizing oxygen delivery seems a worthy goal, studies using a pulmonary artery catheter to set a specified goal (>500 mL/min) thus far have not convincingly demonstrated improved outcomes, although these studies were primarily of unselected rather than specifically ill populations.61,62 Vascular surgery patients with abnormally low oxygen delivery or high oxygen consumption have not been definitively studied. As a practical matter, patients with abnormal oxygen delivery and low mixed venous saturations should have cardiac output or hemoglobin concentration or both optimized.
Table 40-3
Oxygen Measurements
| Measurement | Equation |
| Arterial oxygen content (CaO2) | CaO2 = (1.36 × [Hb] × SaO2) + (PaO2 × 0.0031) |
Mixed venous oxygen content (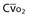 ) ) | 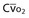 = (1.36 × [Hb] × = (1.36 × [Hb] × 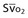 ) + ( ) + (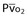 × 0.0031) × 0.0031) |
| Arteriovenous oxygen difference (a-vDO2) | a-vDO2 = CaO2 − CvO2 |
| Oxygen delivery (DO2) | DO2 = ([1.36 × [Hb] × SaO2] + [PaO2 × 0.0031]) × CO |
Mixed venous oxygen saturation (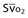 ) ) | 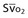 = (1.36 × [Hb] × SaO2) + ( = (1.36 × [Hb] × SaO2) + (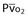 × 0.0031) × 0.0031) |
Oxygen consumption ( ) ) | VO2 = CO × (a-vDO2) |
CO, Cardiac output; Hb, hemoglobin.
Clinical Presentation
Atelectasis
Clinically significant atelectasis has been reported in 20% of patients undergoing upper abdominal surgery and in 30% of patients undergoing thoracic surgery.59,60,63 Altered compliance of lung tissue, impaired regional ventilation, and retained airway secretions contribute to the development of atelectasis.2
Nosocomial Pneumonia
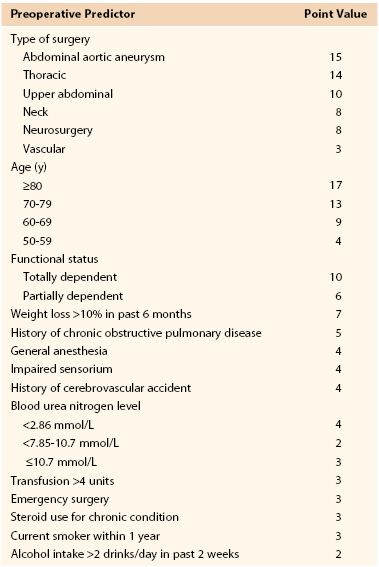
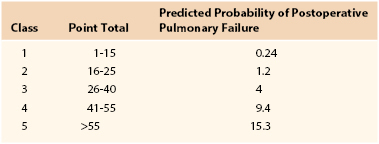
Nosocomial pneumonia is the leading cause of death due to hospital-acquired infections. Estimates of associated mortality range from 5% to 50% and depend greatly on the degree of case-matching and controls.64–66 The greatest risk factor is duration of mechanical ventilation; an average risk of ventilator-associated pneumonia is 1% per day, although the daily risk of acquisition of ventilator-associated pneumonia is highest during the first week of intubation (Box 40-2). Arozullah et al8,67 developed an index to predict the risk of postoperative pneumonia using a large Veterans Administration database and modeled it after their pulmonary risk index. They assigned each factor points on the basis of its strength in a multivariate analysis (Table 40-4). Again, procedure-related risk factors dominated the index; however, it also did identify several modifiable risk factors, including functional status, weight loss, cigarette use, and alcohol consumption.
Microaspiration is thought to play a central role in the pathogenesis of nosocomial pneumonia and is discussed further in a separate section.41,42 Nosocomial pneumonias are frequently polymicrobial, with gram-negative bacilli predominating in 60% of nosocomial pneumonias and constituting six of the seven most frequently identified pathogens.68–70 In some institutions, Acinetobacter species can be important pathogens. Large variations in pathogens may be seen within and between institutions, and clinicians should be familiar with the specific pathogens and sensitivities in their own institutions and especially in ICUs.
Diagnostic strategies and controversies regarding nosocomial pneumonia are beyond the scope of this chapter. Suffice it to say that nosocomial pneumonia is often diagnosed on uncertain clinical grounds and treated although not clearly present by histologic grounds.71 Whether an aggressive invasive strategy employing bronchoalveolar lavage should be used for diagnosis remains a subject of active debate. A study from France evaluated an invasive diagnostic approach using protected brush specimens or bronchoalveolar lavage versus clinical criteria in 413 ICU patients with a clinical suspicion of ventilator-associated pneumonia and noted significantly lower mortality at 14 days (16% vs 26%) and 28 days as well as fewer antibiotic days and lower number of antibiotics administered.71 However, a study by the Canadian Clinical Trials Group suggested that there are no significant differences in outcomes based on whether an invasive or noninvasive method of diagnosis of ventilator-associated pneumonia is used.72
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree