Chapter 39
Systemic Complications
Cardiac
William Scott Beattie, Giora Landesberg
In an editorial in 2004, Mosucuci and Eagles wrote, “If one carefully screens vascular patients and excludes patients with symptoms of unstable angina, left main disease, aortic stenosis, and severe left ventricular dysfunction, and if one provides excellent medical care to those remaining, then coronary artery revascularization does not appear to provide additional benefit.”*
Epidemiology
Prevalence of Coronary Disease
Atherosclerosis is ubiquitous in vascular surgical patients. Hertzer drew attention to this by showing the small percentage of vascular surgery patients (8%) who were free of coronary artery disease (CAD).1 This study, conducted over 30 years ago, revealed that 5% of vascular surgical populations at the Cleveland Clinic had left main CAD and that 11% had significant three-vessel disease. Notably, in our opinion, the most important finding was that 15% of patients had severe, yet unsuspected, coronary disease.
The intervening 30 years have seen seismic changes in both the primary and secondary prevention of CAD, including smoking cessation, the adoption of aggressive lipid-lowering therapies, and a 30% decrease in the incidence of myocardial infarction and cardiac death in nonsurgical populations.2 Conversely, patients presenting for vascular surgery in 2013 are on average a decade older and have more comorbidities, and the incidence of major coronary pathology in vascular patients may be higher. Thus despite clear advances in the treatment of CAD, it is conceivable that more patients presenting for vascular surgery have silent yet significant coronary artery disease. This paradox is illustrated in the Coronary Artery Revascularization Prophylaxis (CARP) trial, in which 17% of all patients who underwent angiography had three-vessel disease and an additional 4.6% had left main disease.3 In the intervening years, our attempts to identify patients with significant coronary disease, through a variety of processes of care, have apparently been inadequate. This was demonstrated by Monaco et al, who screened patients using the American Heart Association/American College of Cardiology (AHA/ACC) guidelines and identified fewer patients with three-vessel or left main coronary disease than a randomized cohort having routine preoperative coronary angiograms.4
Our current processes of care fail to identify many patients with significant CAD, and when we do correctly identify them, few effective treatments are available. In this chapter we will outline and define the pathology, incidence, diagnostic criteria, risk stratification, and treatment options designed to limit the effect of postoperative cardiac adverse events.
Cardiac Complications
Definition of Adverse Cardiac Events
We suggest that only the terms myocardial infarction (MI), congestive heart failure (CHF), cardiac arrest, and death have utility in the modern lexicon of adverse cardiac events.
Myocardial Infarction
MI is the leading cause of death after surgical procedures. The Third Universal Definition of MI,5 as endorsed by all major cardiology organizations, is a rise and fall in a cardiac biomarker, mainly cardiac troponin, with at least one measurement above the 99th percentile of the upper reference limit (for the specific troponin assay), associated with at least one of the following:
2. Electrocardiographic evidence of ischemia, ST-T wave changes or left bundle branch block.
3. Development of pathologic Q waves.
4. Imaging showing loss of viable myocardium or a new abnormal LV wall motion.
The universal classification of myocardial infarction is outlined in more detail in Box 39-1.
If this low cutoff level of troponin elevations is used to define MI, postoperative myocardial injury occurs in as many as 25% of vascular surgery patients. Myocardial infarction comprises two distinct electrocardiographic entities. The first subgroup, those patients who may have ST-elevation MI (STEMI), accounts for less than 1% of surgical patients.6 The second, and largest, subgroup sustain a biomarker rise with ST-segment depression or without transient ST-T changes, which is analogous to a non–ST-elevation myocardial infarction (NSTEMI).
Congestive Heart Failure
CHF is ill defined in older investigations and is frequently the result of fluid overload or transfusion-associated cardiac overload (TACO) (as prime examples). However, the inability to handle excess fluids is a sign of a failing left ventricle. We would submit, therefore, that in this modern era of advanced echocardiographic technology and training, postoperative CHF should be more thoroughly investigated with biomarkers, chest radiography, and cardiac imaging (i.e., echocardiography).
Cardiac Death
Death sometimes occurs before biomarkers are measured (or appear), and therefore the following situations have been defined as cardiac deaths: (a) death that is sudden and unexpected, (b) cardiac arrest with symptoms suggestive of myocardial ischemia, (c) death that is accompanied by new ST elevation or new left bundle branch block (but before a biomarker can be obtained), and (d) autopsy evidence of fresh thrombus in a coronary artery.
Perioperative Myocardial Injury
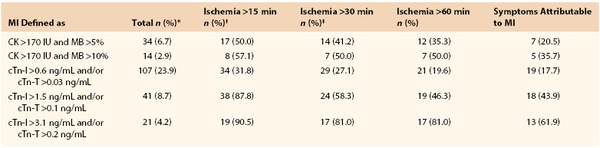
A discussion on myocardial injury is necessary since many patients with postoperative troponin elevations do not fulfill the additional diagnostic criteria necessary for the diagnosis of postoperative MI. A secondary analysis of POISE trial makes clear that postoperative MI is predominately clinically silent.6 ST-T changes are captured in less than 30% of the patients, and many patients have cardiac biomarkers detected that are totally asymptomatic. Levy and colleagues conducted a meta-analysis of seven studies where a biomarker has been used to routinely screen all patients, which draws attention to the association between elevated cardiac biomarkers and postoperative death.7 In the intervening 2 years there have been three more evaluations in which vascular patients have had universal biomarkers drawn postoperatively.8–10 These 10 studies found that 1,032 of the 6,083 patients had significant troponin elevations (16.3%). Similarly, in FOCUS, a randomized trial assessing transfusion triggers in elderly orthopedic patients with universal biomarker monitoring, only 33% of patients with detectable biomarker had MI.11 Landesberg et al showed essentially the same results in vascular surgical patients (Table 39-1).12
The reasons for this lack of symptomatology remains speculative but may include the concomitant use of narcotics, diabetes mellitus, competing somatic sensations, and a high incidence of symptoms such as postoperative nausea and vomiting, which coexist with both surgery and acute cardiac ischemia. The lack of ECG changes may be due to the transient nature of postoperative ST-T changes and the lack of routine cautious monitoring of postoperative ECG with ST-segment analysis.15 Furthermore, ST-segment monitoring may be complicated by preexisting left or right bundle branch blocks, baseline ST-T changes, drug effects, acid/base alterations, and electrolyte abnormalities.16,17 In addition, without regular surveillance, as many as two thirds of biomarker elevations will be missed, which underlines the importance of ordering troponin in high-risk patients in the postoperative period.
Definition of Myocardial Infarction
The universal classifications of myocardial infarction (see Box 39-1) distinguish between two main types of MI10:
The other types of MI in the universal definition are less relevant to this discussion and include sudden cardiac death before biomarkers are available (type 3) or as a result of coronary interventions (post-PCI—type 4; post CABG—type 5).
Type 1 MI
This type typically occurs as a result of an unstable or “vulnerable” coronary plaque and is characterized by a large lipid core and a thin, weakened fibrous cap, which suddenly undergoes acute disruption, leading to acute coronary thrombosis. Type 1 MI, together with unstable angina pectoris or sudden cardiac death, is defined as acute coronary syndrome (ACS).
When plaque rupture and coronary thrombosis occur in a major coronary artery, supplying an area that was not significantly stenosed before the acute occlusion and therefore lacks sufficient collaterals, the result is often ST-segment MI (STEMI). As a rule, STEMI, is a medical emergency causing severe chest pain, dyspnea, and other related symptoms, requiring immediate intervention to reopen the occluded coronary artery, preferably by percutaneous coronary intervention (PCI) or by thrombolysis. Even with immediate coronary intervention, STEMI is associated with up to 10% early mortality.
Other patients may have similar symptoms but no ST-segment elevations or rather ST-segment depression on ECG; these infarctions are defined as non–ST-segment MI (NSTEMI). Pathologically, these may be plaque ruptures and coronary thromboses in coronary arteries supplying smaller myocardial territories or territories supplied also by collaterals or thromboses causing nontotal coronary occlusions. Patients with NSTEMI usually do not require—and do not benefit from—immediate coronary interventions, unless hemodynamic instability or progression of symptoms occurs.
In the past, in nonsurgical Western populations, the proportions of STEMI and NSTEMI were approximately 50% each. More recently, the absolute incidence of STEMI is decreasing in the West while the incidence of NSTEMI is consistently increasing. Reasons for this shift include the older average age of the Western population, increased availability of primary and secondary prevention strategies, and better detection of NSTEMI through high-sensitivity troponin assays. Hence STEMIs currently account for 30% or less of all myocardial infarctions.
Type 2 MI
In contrast, type 2 MI typically occurs in patients with severe yet stable CAD, usually the result of extracardiac causes leading to a prolonged imbalance between myocardial oxygen supply and demand. The most common triggers for type 2 MI are prolonged tachycardia, hypotension or hypertension, anemia, and possibly, emotional or physical stress causing coronary vasospasm.
Type 2 MI is almost uniformly associated with ST-depression or non–ST-elevation ischemia and is therefore indistinguishable from type 1 NSTEMI. The circumstances (i.e., spontaneous in type 1 versus stress-induced in type 2) should help differentiate between the two types of MI. Current data suggest that the vast majority of perioperative myocardial infarctions are NTSEMI and most likely, type 2.
Cardiac Biomarkers
Several biomarkers have been developed to measure myocardial injury. Currently, the most frequently used biomarker is cardiac troponin cTn (T or I), followed by the myocardial band of creatinine kinase (CK-MB). Other less frequent tests include lactate dehydrogenase (LDH), aspartate transaminase (AST), myoglobin ischemia modified albumin, glycogen phosphorylase isoenzyme BB and pro-brain natriuretic peptide. Since troponin assays are based on monoclonal antibodies specific to the cardiac troponins (I and T), they are the most sensitive and specific tests for myocardial injury, whereas CK-MB is specific only when there is no skeletal muscle damage.
The remaining tests lack specificity since other causes of tissue damage can elevate the marker. LDH is a late occurring marker of injury (normalizing in 10-14 days) that can also be elevated in cancer, meningitis, or human immunodeficiency virus (HIV). AST is primarily a liver function test. Myoglobin, the main intracellular oxygen transport molecule in myocytes, lacks specificity since it is released in all forms of muscle degradation; yet it has the advantage of the earliest detection after injury (2 hours) and is sometimes used to monitor reperfusion.
Postoperative Troponin Elevations and Mortality.
Troponin elevation in the first 3 days after major vascular surgery is incrementally associated with higher 5-year mortality.14 Recently, VISION trial reported that myocardial injury, as measured by a fourth generation high-sensitivity troponin T assay in the first 3 days after major general surgery, shows “dose response” association between the degree of troponin detected and 30-day postoperative mortality.13 A single-center retrospective study showed that when troponin measurement is not a standard of care, myocardial injury is missed in as many as 66% of patients.9 This study also showed that isolated cardiac troponin release is associated with increased mortality in a “dose-dependent” manner. Levy et al conducted a meta-analysis of seven studies in which cardiac troponin has been used to routinely screen all patients and demonstrated the association between elevated cardiac troponins and postoperative death.7 The incidence of increased cardiac troponins in these studies ranged from 12.4% to 26.5%. It is recommended that serum troponin concentrations be measured in high-risk vascular surgery patients (we suggest that this be defined as a Revised Cardiac Risk Index ≥2) for the first 3 postoperative days.
Electrocardiography
The electrocardiogram occupies a central position in the diagnosis of myocardial infarction, as is seen in the recently revised consensus statement, the Third Universal Definition of Myocardial Infarction.5 Acute ST-segment changes indicate MI and, in conjunction with troponin elevations, can indicate evolving infarction. However, in the presence of preexisting changes, such as left or right bundle branch block, pacemaker rhythms, or baseline ST-T wave changes, these diagnostic criteria become less specific and less sensitive. This is particularly problematic in vascular surgery, in which more than 30%18 of patients demonstrate such changes. This becomes even more troublesome when one considers that the incidence of these preexisting electrocardiographic changes increases with the underlying cardiac risk.
Objective measurements such as continuous 12-lead ECG recording in the perioperative period, combined with rigorous screening of cardiac biomarkers, might give a more accurate estimation of the true incidence of perioperative MI. A few studies have used this modality, all of them in patients undergoing major vascular procedures. One of these studies found that in 447 patients scheduled for elective open abdominal aortic repairs and monitored by continuous 12-lead ECG recording, cardiac troponin, and CK/CK-MB for the first 3 postoperative days, 23.9% of patients experienced cardiac troponin release above the lowest cutoff level. Remarkably, fewer than a third (31.8%) of these patients had perioperative ST-segment changes (see Table 39-1).12
Cardiac Imaging
We are unaware of any perioperative studies that have evaluated either left ventricular function, new onset regional wall motion abnormailties, or mitral insufficiency to aid in the diagnosis of MI. Many imaging modalities are difficult to conduct postoperatively. However, with the advent of bedside ultraound and limited cardiac echocardiography, this may change in the near future. Focused cardiac ultrasound examinations have been show to improve the diagnosis of MI in Emergency Departments.19
Pathogenesis of Perioperative Myocardial Infarction
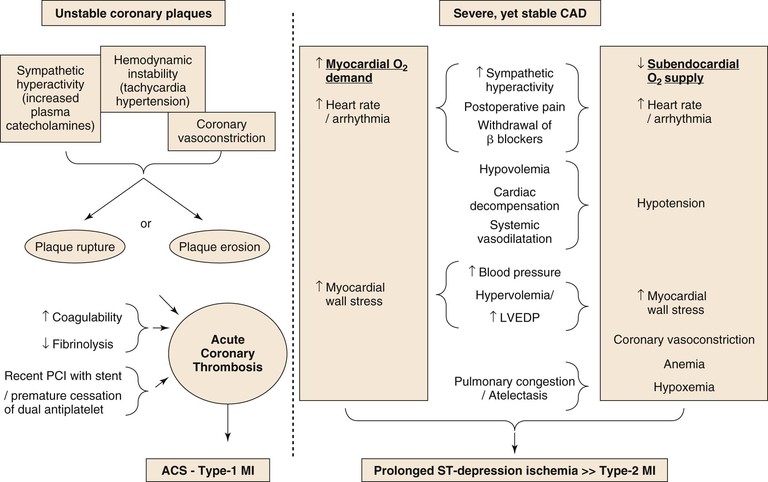
A schematic diagram of proposed perioperative myocardial injury mechanisms is seen in Figure 39-1. The perioperative surgical stress response includes a catecholamine surge with associated hemodynamic stress, vasospasm, reduced fibrinolytic activity, platelet activation, and consequent hypercoagulability. However, despite the common occurrence of CAD in vascular surgery patients, STEMI is rare compared with the common occurrence of NSTEMI, suggesting that perioperative MI is most commonly caused by a sustained imbalance between myocardial oxygen supply and demand, usually as a result of the tachycardia and increase in myocardial load.20
Episodes of perioperative ST-segment depression, indicative of subendocardial myocardial ischemia, have been described in up to 41% of vascular surgery patients, mostly occurring within the first 2 days after surgery,21–23 whereas Q wave infarction is rare.6,24 Although plaque disruption fissure or hemorrhage can be found in postmortem analyses in up to half of patients who died after postoperative MI,25,26 similar findings are also common in patients with CAD who die from other, noncoronary causes (see Fig. 39-1).
Incidence of MI after Vascular Surgery
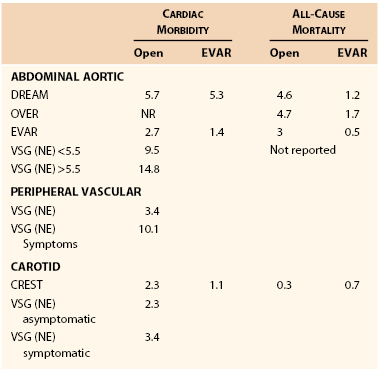
The incidence of MI after vascular surgery has been reported in various formats, including prospective randomized trials, prospective observational trials, and retrospective reports. The incidence of major cardiac complications is dependent on patient characteristics, symptomatology, and type of surgery. The incidence of major cardiac complications is displayed in Table 39-2.
Aortic surgery has the highest incidence of cardiac morbidity, ranging from 1.4% in the OVER trial to more than 14% in the Vascular Study Group of New England registry.27 Generally, the event rates in randomized trials are half of those in registries. In addition, the cardiac event rate is higher in patients with larger aneurysms. The acute in-hospital infarctions rates are lower for endovascular compared to open procedures. 28–31 The cardiac event rate in peripheral vascular surgery is dependent on symptoms; patients with peripheral ischemic symptoms have more than a 10% cardiac event rate, which is three times that of patients without rest ischemia.
Carotid surgery is associated with approximately 2% to 3% perioperative cardiac morbidity.32–34 The results from randomized trials are similar to those from registry data. Finally, patients with symptomatic carotid disease have similar rates of MI based on the registry data. Carotid endarterectomy is associated with a doubling in cardiac morbidity for surgery as opposed to a carotid stent procedure; however, this was more than offset by an increased stroke rate in patients having stenting procedures.35,36
Preoperative Cardiac Evaluation
Three decades ago, based on their observations of the high incidence of severe CAD in patients undergoing major vascular surgery, Hertzer et al established the philosophy that the mere fact that a patient has atherosclerosis and requires vascular surgery justifies a high index of suspicion, which may lead to coronary investigation and possible revascularization.1
Since then, tremendous changes have occurred in the field, which have led to our current practices based on patient risk,37 in which coronary investigation and revascularization are selectively performed before major vascular surgery. The changes in this philosophy and the approach to the cardiac patient undergoing major surgery include the following.
2. Numerous advances in cardiology have tempered the enthusiasm for prophylactic revascularization.
• Percutaneous coronary intervention does not improve survival in patients with stable CAD.38
The following paragraphs outline the major historic developments in the field in the last decades, along with the most important tools that are currently available for preoperative cardiac investigation. A careful attempt will be made to provide general recommendations for preoperative cardiac assessment and intervention. Mosucuci and Eagle, in an editorial accompanying the CARP trial in 2004, commented,
“The issue of whom to screen and how to screen preoperative patients beyond history taking, physical examination and preoperative electrocardiogram is far from settled.”1
Preoperative cardiac risk assessment affects perioperative decision making in three ways. (1) Given the patient’s anatomical and/or symptomatic indication for vascular surgery, a high cardiac risk may influence the perioperative or long-term risk-benefit ratio of the elective surgery and may therefore lead to its deferral. (2) Effective cardiac risk assessment may enable preoperative optimization by prompting medical therapy for cardiac failure (if present); in specific cases, risk assessment may lead to preoperative coronary interventions to reduce perioperative and long-term cardiac risk. (3) Accurate preoperative knowledge of the patient’s cardiac function and risk allow better intraoperative and postoperative treatment and resource utilization, such as intraoperative monitoring and postoperative ICU monitoring, when needed, for the patient.
Cardiac Risk Indices
As a general rule any risk index must be simple, accurate, and generalizable across populations. Numerous clinical risk indices have been proposed, including the seminal Goldman cardiac risk index,39 the Detsky’s risk index,40 l’Italien,41 the Glasgow Aneurysm Score,42 and others. However, no index has yet gained wide and lasting acceptance.
In 1999 Lee et al constructed a simplified revised cardiac risk index (RCRI).37 In a derivation cohort of 2893 consecutive patients, six independent risk factors were identified: (1) a history of heart disease, (2) a history of congestive heart failure, (3) cerebral vascular disease, (4) insulin therapy, (5) chronic renal failure (defined as a preoperative creatinine of 177 mmol/L, or 2 g/L), and (6) high-risk surgery (major vascular, thoracic, and upper abdominal surgery). The validation cohort of 1422 patients showed that patients with none, 1, 2, and 3 of these factors had cardiac complications of 0.4%, 0.9%, 7%, and 11%, respectively. The overall accuracy ranged between 0.75 and 0.80 and was superior in this population to the other cardiac risk indices and the American Society of Anesthesiologists (ASA) classification. Since its publication, the RCRI has been widely used and adopted by the ACC/AHA guidelines committee for the preoperative stratification of patients’ perioperative cardiac risk.43 The index has also been shown to be generalizable across numerous populations.
A recent meta-analysis of 24 studies in over 750,000 patients shows that the RCRI discriminated moderately well in mixed surgical populations, with a sensitivity of 0.65 and specificity of 0.76.44 When this meta-analysis was limited strictly to studies of vascular surgical populations, the RCRI had an aggregate sensitivity and specificity of 0.70 and 0.55, respectively. The vascular surgery group has also published a modification of RCRI that purports to improve the prediction of major cardiac events.27 However, this analysis suffers from the fact that not all patients were screened for a biomarker elevation. This methodologic defect has been shown to underestimate the incidence of MACE by up to threefold.9 A recent analysis of the accuracy of the RCRI in vascular surgical patients, who also had universal biomarker screening, had calculated receiver operating characteristic of 0.75.45 Therefore it is our opinion that the RCRI alone is inadequate to reliably screen vascular surgery patients; it was proposed that further information be gained through laboratory testing to increase the accuracy of the preoperative cardiac investigation.
Preoperative Laboratory Testing
Complete Blood Count
Laboratory findings are used routinely for preoperative risk assessment, and the most frequent lab test in the preoperative assessment is a complete blood cell count. Preoperative anemia has been linked to increasing cardiac morbidity.45 Preoperative anemia is also linked to increased transfusion requirements, which have been secondarily shown to increase cardiac morbidity. In particular, surgical patients who regularly take beta blockers do not tolerate acute anemia, which may lead to increased cardiac morbidity.46 We are unaware of any study that has incorporated anemia into a risk index.
Creatinine
The RCRI shows that chronic renal failure is independently associated with increased cardiac morbidity. However, creatinine is closely related to lean muscle mass. Thus many elderly patients with normal creatinine levels have chronic renal failure. The conversion of creatinine to glomerular filtration rate, through either the Modification of Diet in Renal Disease Study or the Cockcroft-Gault equations may increase the sensitivity and specificity of the measurement.47
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree