Essentials of Diagnosis
General Considerations
Syncope can be defined as a sudden, transient loss of consciousness and postural tone that fully resolves spontaneously without specific intervention (eg, cardiopulmonary resuscitation [CPR], electrical or chemical cardioversion). The common pathophysiologic mechanism responsible for most syncopal spells is a transient reduction in cerebral blood flow and cerebral hypoperfusion. Reduced cerebral blood flow from cardiovascular and neurocardiogenic causes accounts for most cases in which a diagnosis can be made. Even when cerebral blood flow is normal, a reduced delivery of such essential cerebral nutrients as oxygen and sugar can occasionally cause altered consciousness.
Syncope is a common condition experienced by up to 50% of adults in a lifetime. It is responsible for about 3% of hospital admissions and 4% of emergency department visits. Physicians are frequently consulted to evaluate this symptom and—more commonly—presyncope, dizziness, or light-headedness, which may have a similar pathogenesis.
Syncope has many causes (Table 16–1), most of which have a benign prognosis. Because cardiac causes are associated with greater morbidity and mortality, early recognition of structural heart disease or other cardiogenic causes is important in order to prevent sudden death or injury.
Cardiac |
Obstruction to blood flow |
Valvular stenosis |
Hypertrophic cardiomyopathy |
Prosthetic valve dysfunction |
Atrial myxoma |
Congenital heart disease |
Pericardial tamponade |
Pulmonary hypertension |
Pulmonary emboli |
Pump failure (myocardial infarction or ischemia) |
Arrhythmias (decreased cardiac output) |
Bradyarrhythmias |
Sinus bradycardia |
Sick sinus syndrome |
Atrioventricular block (Adams-Stokes attacks) |
Pacemaker malfunction |
Drug-induced bradyarrhythmia |
Tachyarrhythmias |
Ventricular tachycardia |
Supraventricular tachycardia |
Torsades de pointes |
Neurocardiogenic |
Vasovagal |
Orthostatic |
Carotid sinus hypersensitivity |
Situational (tussis, micturition, defecation, deglutition) |
Other Causes |
Seizures |
Drugs |
Hypoglycemia |
Hypoxia |
Hypovolemia |
Cerebral vascular insufficiency |
Extracranial vascular disease |
Neuralgia |
Psychogenesis |
Hyperventilation |
Pathophysiology & Etiology
Any obstructive structural lesion of the left or right side of the heart can critically reduce the cerebral blood flow. Exertional symptoms are common with obstructive lesions because cardiac output does not rise normally with exercise and cerebral perfusion is not maintained. Obstruction to left ventricular outflow occurs with aortic valve stenosis, mitral stenosis, left atrial myxoma, prosthetic aortic or mitral valve dysfunction, and hypertrophic cardiomyopathy. The ventricular arrhythmias that can occur with valvular heart disease may be responsible for both exertional and nonexertional syncope as well as sudden death.
Lesions that obstruct flow through the right side of the heart include right atrial myxoma, pulmonary stenosis, tricuspid stenosis, pulmonary hypertension, and pulmonary emboli. Limitations to right ventricular outflow diminish the cardiac output and the ability to increase the output with exertion. Exertional syncope is common with severe pulmonary hypertension and severe pulmonic stenosis.
Nonexertional syncope can be the result of pulmonary emboli (hypoxia and obstruction of right ventricular outflow) and aortic dissection with pericardial tamponade, which impedes right ventricular filling and decreases cardiac output.
Both bradyarrhythmias and tachyarrhythmias can cause transient decreased cardiac output with resultant cerebral hypoperfusion. Bradycardias result in symptoms when the rate is so slow that the compensatory increase in stroke volume is inadequate to maintain blood pressure. Periods of ventricular asystole as short as 5 seconds can cause syncope (from the ensuing cerebral hypoperfusion). Mechanisms of symptomatic ventricular bradycardia and asystole include sinus node disease (sinus exit block, sick sinus syndrome, marked sinus bradycardia, or sinus arrest) and second- or third-degree atrioventricular (AV) block. Syncope from Mobitz II second-degree AV block with paroxysms of several consecutive P waves that fail to conduct to the ventricle is called an Adams-Stokes attack. Medications can also cause syncope, and any patient with syncope from bradycardia must be thoroughly questioned regarding medications. Calcium channel blockers, digoxin, β-blockers (including optical formulations), sympatholytics, primary antiarrhythmics, and other medications can all decrease heart rate and increase AV block—enough to cause symptoms in susceptible patients.
Pacemaker malfunction is another possible cause. If a pacemaker was previously implanted for sinus node disease or high-degree AV block and the patient has a recurrence of syncope, there may be a pacemaker system problem, such as battery failure or lead fracture.
Transient decreased cardiac output during ventricular or supraventricular tachycardias results when the ventricular rate is fast enough to decrease diastole significantly and thus decrease ventricular filling (preload). Concomitant peripheral vasodepression with resultant hypotension may also play a role in the pathophysiology of syncope with supraventricular arrhythmias.
Ventricular tachycardia occurs most frequently in patients with organic heart disease, particularly coronary artery disease with previous myocardial infarction. Ventricular tachycardia is an important and ominous cause of syncope because ventricular tachycardia usually precedes ventricular fibrillation. Symptoms and prognosis are related to the degree of underlying myocardial dysfunction and the rate and duration of the arrhythmia.
Supraventricular arrhythmias are more likely to cause palpitations and presyncope than true syncope. Although they occur often in young patients with structurally normal hearts, they are also prevalent in structurally abnormal hearts. As with ventricular arrhythmias, the severity of the symptoms is related to the ventricular rate of the arrhythmia and the degree of underlying myocardial dysfunction. Mechanisms associated with syncope include atrial arrhythmias (fibrillation, flutter, tachycardia) with rapid ventricular response, AV nodal reentrant tachycardia, and supraventricular arrhythmias associated with accessory pathways.
Torsades de pointes is a rapid polymorphic ventricular arrhythmia classically known to cause syncope in association with class I antiarrhythmic drugs. Because this arrhythmia can also cause sudden cardiac death, it is very important to understand the reversible conditions that can precipitate it. Most cases of torsades de pointes are associated with acquired long QT syndromes that can occur with several drugs (class Ia and III antiarrhythmics, erythromycin, certain antihistamines, and tricyclic antidepressants), electrolyte abnormalities (hypomagnesemia, hypokalemia, and hypocalcemia), myocardial ischemia, and central nervous system disorders. Rarely, long QT syndrome occurs congenitally, in hereditary forms of prolongation of the QT interval (with or without associated deafness).
This is the most common mechanism of syncope in young, otherwise healthy individuals, constituting approximately 60% of cases. The underlying mechanism is a paradoxical autonomic reflex resulting in profound hypotension (vasodepression) that is often associated with bradycardia (vasovagal). The underlying pathophysiology of vasovagal syncope has been elucidated with the aid of tilt-table testing (see the section on Diagnostic Studies). This technique provides a controlled means of reproducing vasovagal syncope and serves as a means to guide medical therapy. Before the development of tilt-table testing, the diagnosis of vasovagal syncope was largely a matter of exclusion.
Despite extensive study by numerous investigators, several controversies still remain regarding the underlying pathophysiology of vasovagal syncope. When one stands, a significant amount of venous pooling occurs, which can shift from 300 to 800 mL of blood away from the central circulation. To counteract this gravitational effect, baroreceptors in the carotid sinus, aortic arch, and left ventricle sense decreased stretch (low pressure) and send afferent neural messages to the vasomotor center in the brainstem’s medulla, which in turn sends efferent stimuli to increase peripheral sympathetic and cardiac tone (vasoconstriction and increased heart rate and contractility) and decrease vagal tone. This is the normal neural reflex arc that maintains cerebral arterial pressure and prevents hypotension or syncope with orthostatic changes.
It is believed that this reflex arc goes awry in susceptible individuals, resulting in a mixed response of vasodepression (hypotension) and cardiac inhibition (bradycardia). This symptomatic reflex arc, commonly known as a vasovagal episode, can occur as pure vasodepression or, rarely, pure cardiac inhibition; the classic mixed response is most common, with hypotension often preceding the bradycardia.
Vasovagal syncope is often, but not always, preceded by predisposing factors such as fear, pain, injury, fatigue, prolonged standing, or such medical procedures as venipuncture. During the initial phase, it is believed that blood pressure and heart rate increase in response to circulating catecholamine increase, sympathetic nerve stimulation, and vagal withdrawal. This is abruptly followed by hypotension, bradycardia, and syncope caused by inhibition of sympathetic vasoconstrictor and cardiac stimulatory activity and increases in vagal tone.
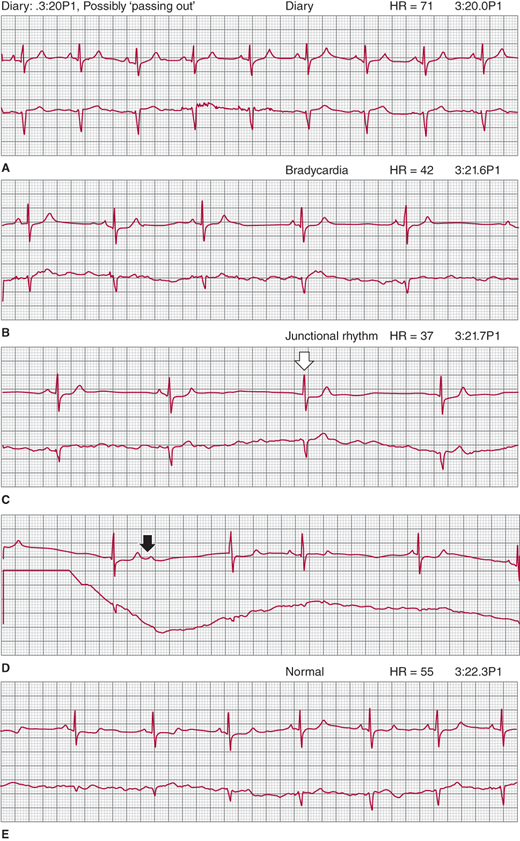
Although the exact pathophysiology is not understood, two conditions are thought to bring on the paradoxical reflex in susceptible individuals: reduced left ventricular filling (an empty ventricle) from decreased venous return and increased left ventricular pressure from increased contractility caused by cardiac stimulation from circulating catecholamines. A vigorously contracting empty ventricle is thought to paradoxically increase left ventricular pressure (mimicking hypertension), which is sensed by left ventricular stretch receptors (C fibers). This afferent neural arc to the nucleus tractus solitarius in the medulla results in an efferent arc that paradoxically decreases sympathetic and increases vagal tone, causing hypotension and bradycardia, respectively. Because both the sinus node and AV node are richly innervated with autonomic nerves, bradycardia can be caused by both vagal depression of sinus node automaticity and decreased AV nodal conduction with AV block. Assuming a supine posture provides spontaneous resolution because the gravitational effects on cerebral blood flow are immediately neutralized. Figure 16–1 shows a two-lead ambulatory recording of a classic vasovagal episode in a patient with previously undiagnosed syncope.
Figure 16-1
Consecutive two-lead rhythm strips recorded from a patient wearing an ambulatory monitor during a vasovagal near-syncope spell, illustrating the classic response. A: In his diary, the patient indicates that he is possibly passing out. Because heart rate and rhythm are normal, the symptom is due to initial hypotension from vasodepression. B: As the episode proceeds, sinus bradycardia develops from vagal cardiac inhibition. C: Later still, sinus rhythm is completely suppressed, resulting in a junctional escape rhythm; open arrow shows first junctional beat. D: Vagal inhibitory effect on the atrioventricular node is also shown because a sinus beat did not conduct to the ventricle (solid arrow). E: Complete resolution to normal sinus rhythm, correlated with spontaneous full recovery.
Vasovagal syncope is usually considered a benign form that can often be treated with patient education and avoidance of the precipitant. Some individuals, however, have frequent severe spells that result in injuries and, rarely, sudden death. Several therapies (discussed later) are available for patients who require intervention.
The carotid sinus has baroreceptors located in the internal carotid artery just above the bifurcation of the common carotid artery. These are sensitive to stretch and pressure and give rise to afferent impulses to the vasomotor center in the medulla. In susceptible individuals, particularly the elderly, activation of this reflex results in vagal efferent stimulation, which causes marked bradycardia or AV block (the cardioinhibitory response) with vasodepression (from efferent sympathetic inhibition). In contrast to vasovagal syncope, the cardioinhibitory response is usually dominant. Syncope from carotid sinus hypersensitivity usually is suggested by historical factors: precipitation with turning the head, shaving the neck, or wearing a tight shirt collar. Spontaneous attacks can also occur. Full consciousness is usually regained in less than a minute after attaining a supine position. When syncope from carotid sinus hypersensitivity is suspected, carotid sinus massage (CSM) can be used to diagnose this condition; hypersensitivity is generally defined as cardiac asystole of 3 seconds or more or a decline in systolic blood pressure of at least 50 mm Hg.
The pathophysiologic mechanisms of syncope associated with micturition, defecation, coughing, and swallowing are not precisely elucidated. It is suggested that the vagal-sympathetic autonomic reflex (hypotension and bradycardia) is involved, with afferent neural stimulation coming from the involved viscera. The Valsalva maneuver (used during micturition, defecation, and coughing) is thought to contribute to hypotension by decreasing venous return, which decreases cardiac output.
In micturition syncope, which classically occurs on waking, a combination of physiologic changes that occur during sleep (eg, sudden decompression of the bladder, decreased heart rate and blood pressure) may contribute to syncope. Tussive syncope classically occurs in middle-aged men who drink alcohol, smoke, have chronic lung disease, and experience paroxysms of severe cough; it is thought to be largely due to decreased venous return caused by the Valsalva-like action of coughing. Deglutition syncope is believed to be entirely due to dysfunction of the afferent-efferent reflex arc and is associated with structural abnormalities of the esophagus (eg, diverticula, diffuse esophageal spasm, achalasia, stricture) and heart (eg, acute rheumatic carditis, myocardial infarction).
Volume depletion, medications, and autonomic dysfunction (primary and secondary) can all lead to cerebral hypoperfusion and syncope in the standing position. Primary chronic autonomic dysfunction is associated with Shy-Drager syndrome, Parkinson disease, and other rare dysautonomias. Secondary autonomic dysfunction with syncope has been described with diabetes mellitus, anemia, amyloidosis, multiple sclerosis, and human immunodeficiency virus (HIV) infection. Medications that commonly cause orthostatic hypotension include diuretics, antihypertensives (eg, angiotensin-converting enzyme [ACE] inhibitors, β-blockers, calcium channel blockers), and ethanol.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree