Surveillance and Remedial Procedures After Aortic Endografting
Anthony W. Lee
Surveillance is a critical (and possibly the most important) component to the overall treatment strategy following aortic endografting. It is predicated on the assumption that the natural history after endograft repair is unpredictable and unknown at this time. Therefore, surveillance must be lifelong and without exceptions. One can go so far as to say that endovascular treatment without postoperative surveillance is tantamount to no treatment at all. Because of this, while pre-operative risk and anatomic assessments are important in determining suitability for endovascular abdominal aortic aneurysm (AAA) repair, the practical logistics, economics, and the expected compliance of the patient should be considered in the ultimate decision to recommend endovascular (vs. open surgical) repair.
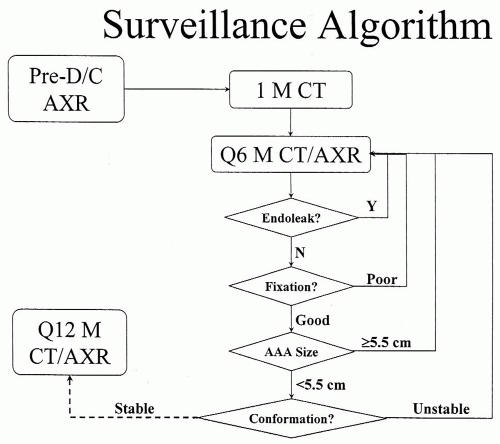
Surveillance Algorithm
There are no uniformly accepted guidelines for surveillance after aortic endografting. In general, however, most use some variation of a schedule involving imaging and office visits at 1-month, 6-month, and 12-month postoperative intervals, followed by 6- to 12-month intervals in the second postoperative year and beyond (Fig. 21-1). Use of an electronic database with an automated mechanism for alerting delinquent follow-up appointments and tracking pertinent longitudinal data can greatly facilitate the management of the sheer volume of data that rapidly accumulates for these patients. Currently, a predischarge computed tomography (CT) scan is rarely performed, as it rarely alters peri-operative management, and the initial postoperative cross-sectional imaging is usually performed at the 1-month visit.
Aneurysm Size
Although its long-term significance is controversial, serial AAA size remains an important surrogate marker of post-endograft success or failure. Currently there are two methods of quantitatively assessing aneurysm size: 2-D diameter and 3-D volume. Regardless of which method is used, the following must be remembered:
The conformation of the aneurysm can change with implantation of the relatively inflexible endograft; therefore, the first postoperative imaging study should serve as the reference for all subsequent measurements.
The aneurysm sac undergoes morphologic changes in three dimensions.
Same imaging modalities should be used to compare any two serial measurements.
Despite software advances in volumetric renderings of CT data, conventional diameter measurements from cross-sectional images remain the “gold standard” for following aneurysm size. These measurements have maintained this role due to their familiarity, availability, and comparability, apart from any issues of software validation or technique. From a technical standpoint, the cross-sectional image of the aneurysm should be conceptually modeled as an ellipse. The size is determined as the largest
pair of measurements (major, minor diameters) obtained from a single image. Interand intra-observer variability of these measurements is usually less than 2 mm. Due to the unpredictable morphologic changes that occur in the postendograft aneurysm, one may find that the slice-level of the image where the maximum diameter is measured may change from one scan to the next, and that increases and decreases in aneurysm size may occur either in the major or minor axis or both. An absolute change in diameter greater than or equal to 5 mm is typically considered clinically significant.
pair of measurements (major, minor diameters) obtained from a single image. Interand intra-observer variability of these measurements is usually less than 2 mm. Due to the unpredictable morphologic changes that occur in the postendograft aneurysm, one may find that the slice-level of the image where the maximum diameter is measured may change from one scan to the next, and that increases and decreases in aneurysm size may occur either in the major or minor axis or both. An absolute change in diameter greater than or equal to 5 mm is typically considered clinically significant.
Endoleaks
The term endoleak refers to any radiographic evidence of a contrast leak or extravasation external to the endograft and within the aneurysm sac. Although the nomenclature for describing endoleaks is evolving along with our understanding of these entities, in general, four types of endoleaks have been commonly recognized and classified as Types I to IV according to their source.
Type I
This refers to a fixation-related endoleak that occurs proximally or distally at the attachment sites. It occurs in less than 5% of all cases and is characterized by an early focal jet of contrast into the aneurysm sac with antegrade flow into the lumbar arteries at completion angiography. For endoleaks coming from the anterior or posterior aspect of the attachment, this identifying jet is obscured by the superimposed endograft. In these cases, a lateral projection can be helpful to visualize its origin. On contrast CT scan, Type I endoleaks have the same Hounsfield attenuation as the adjacent intrastent lumen.
During the early peri-operative period, Type I endoleaks typically signify poor patient selection, case planning, or device implantation. The dogma that one should never leave the operating room with a Type I endoleak is frequently quoted but in reality not always practiced. Longitudinal observation has demonstrated that most Type I endoleaks seal spontaneously within 1 to 6 months. The decision whether to wait or intervene is weighted by the size of the aneurysm and risk of rupture. Obviously, for a smaller aneurysm (<5.5 cm) with a relatively low risk of rupture, it would be reasonable to continue observation, while for larger aneurysms earlier intervention or even elective surgical conversion for a persistent Type I endoleak would be advisable.
Secondary (late or new) Type I endoleaks require immediate investigation and prompt treatment. This is usually due to migration of the main body or an iliac limb of the device from their fixation zone. It represents an acute repressurization of a previously excluded aneurysm sac and may manifest itself as a symptomatic aneurysm. Aneurysms that have shown significant shrinkage after endograft repair return to their pretreatment sizes or even larger.
Type II
Type II endoleaks represent retrograde endoleaks originating from lumbar, inferior mesenteric, accessory renal, or an excluded hypogastric artery. This is the most common type of endoleak and occurs in 20% to 30% of all cases in the immediate peri-operative period. Approximately half of these resolve spontaneously by 1 to 6 months and represent nearly all of the chronic or persistent endoleaks that are in the 10% to 15% of all patients after aortic endografting. During completion angiography, Type II is distinguished from Type I endoleak by a relatively late filling of the aneurysm sac, which is seen after visualization of the branch vessels. On contrast CT scan, Type II endoleaks may sometimes be quite subtle, having a signal attenuation ranging from slightly more than the surrounding mural thrombus to being almost as bright as the endoluminal contrast.
Most Type II endoleaks have a relatively benign natural history. Although the rate of aneurysm shrinkage may be slower or its likelihood decreased in patients with persistent Type II endoleaks, it has not been associated with increased risk of aneurysm rupture or death from rupture. Aggressive endovascular treatment of Type II endoleaks has led to increased secondary intervention rates without obvious impact on aneurysm-related adverse events. Currently the most commonly accepted indication for intervention in Type II endoleak is aneurysm enlargement.
Type III
This refers to a device-related endoleak arising from actual material failure (stent fracture or fabric tear), late component separation, or intercomponent extravasation from inadequate overlapping segments unique to modular (vs. unibody) devices. It carries the same significance as a Type I endoleak in that it represents a direct communication with the aortic circulation and systemic pressurization of the aneurysm and requires prompt management. During completion angiography, it is seen as a contrast jet best seen on selective injections of the suspected limb or endograft body, and on postoperative CT scan, it has the same brightness as the endoluminal contrast.
While an isolated stent fracture from metal fatigue or suture breaks does not necessarily lead to an endoleak, the resulting sharp edge or pointed wire fragment may tear into the graft material, thereby causing the actual endoleak. Improved engineering and routine practice of longer overlapping junctions have greatly reduced the incidence of Type III endoleaks. Once recognized, however, these endoleaks are usually easily treated using endograft cuffs or limbs, and they rarely require surgical conversion.
Type IV
This endoleak refers to the transgraft flow that is sometimes seen in polyester-based endografts due to their intrinsic porosity and suture holes. Interestingly, sutureless expanded polytetrafluoroethylene-based devices do not show a Type IV endoleak, presumably due to their significantly lower porosity as compared to unpreclotted polyester fabrics. A Type IV endoleak is recognized on completion angiography as an early, diffuse blush of the aneurysm sac and resolves within hours of implantation following reversal of the heparin anticoagulation. In rare instances when a suture hole fails to seal, a Type IV endoleak becomes a Type III endoleak as it represents a defect in the actual device.
History and Physical Examination
Interval clinical history involves questions regarding any atypical abdominal or back pain, new-onset claudication, hypertension, or constitutional symptoms of fever or malaise, which may indicate an acute endoleak from device migration, impending endograft limb occlusion, renal artery stenosis, or late endograft infection. Physical examination is focused on aneurysm palpation for pulsatility and femoral pulses. Although it has been shown that persistent pulsatility after endograft repair is unrelated to endoleak, aneurysm shrinkage, or late complications, acute pulsatility in an aneurysm that was previously nonpulsatile may indicate a new Type I or III endoleak.
Imaging
The following are the three main purposes for surveillance imaging:
Detect and characterize endoleaks
Measure aneurysm size
Monitor device integrity and fixation
There are four imaging modalities that may be used for postoperative surveillance of patients after aortic endografting:
Spiral CT angiography
This remains the gold standard for postoperative radiologic surveillance of aneurysm size and endoleak. It is readily available, noninvasive, reliable, and easy to interpret. Sensitivity and specificity for endoleak detection are comparable to or better than ultrasound, magnetic resonance, or conventional angiography, but less for endoleak characterization. Image resolution is excellent at less than 1 mm.
A typical study involves a triple-phase scan consisting of precontrast, contrast, and delayed phases, without oral contrast. It covers the entire abdomen and pelvis from T-12 vertebral body to the femoral heads. The first phase is a noncontrast scan performed at 10-mm slice thickness. The second phase involves a single breath-hold, intravenous timedbolus (150 ml) contrast-enhanced spiral technique at 2.5 to 3.0 mm collimation. The third phase is performed after a 60-second delay from the initial contrast bolus at 10-mm slice thickness. This results in approximately 400 to 500 individual images per study, which are best viewed electronically on a PC or a dedicated PACS workstation, rather than on hardcopies. Corresponding images from all three phases are displayed simultaneously and compared to each other to resolve any areas of unusual signal attenuation. Images should be properly “windowed” (contrast and brightness) to distinguish between contrast-filled lumen, stent, and mural calcium.
The main disadvantages of the CT scan are the contrast and radiation exposure. Contrast becomes problematic for patients with chronic renal insufficiency and a creatinine over 2.5 mg/dl, diabetic patients taking certain oral hypoglycemic agents (e.g., metformin, Glucophage), and contrast allergy. Currently, pretreatment with oral N-acetylcysteine (Mucomyst) or sodium bicarbonate infusion can be used to reduce the incidence of contrast nephropathy in patients at risk.
Four-view abdominal radiograph
This is an inexpensive study that is obtained along with a cross-sectional imaging study. The four views are anteroposterior projection, lateral projection, and two oblique projections. The mA and keV setting should be optimized for metal. More than any other single modality, the plain radiograph affords a bird’s-eye perspective of the overall integrity and conformation of the endograft. Although subtle findings such as small migrations are difficult to discern, gross findings such as large migrations, impending limb separations, endograft conformational changes, and stent fractures can be easily tracked over time to prophylactically intervene as necessary.
Color-flow duplex ultrasonography
This is an important imaging modality that can play a complementary role to a CT scan. It is noninvasive and involves no radiation or contrast. It can reliably measure maximum aortic diameter, detect endoleaks, and identify their origins. Morphologic changes and dimensional relationships near the aortic neck between the endograft and aorta have been more difficult to interrogate with this modality. The quality of the information depends on the patient (e.g., body habitus, excessive overlying gas), equipment, and mostly on the vascular technologist performing the procedure. Consistent and systematic technique, such as aneurysm measurements based on a fixed anatomic reference, is critical for longitudinal assessment and making important treatment decisions based on changes. Recent introduction of ultrasound contrast agents has increased the relative signal-to-noise ratio of the images and ability to detect and characterize endoleaks.
Gadolinium (Gd)-enhanced magnetic resonance angiography (MRA)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree