Approximately 20% to 30% of patients with Noonan syndrome (NS) have asymmetric left ventricular hypertrophy (LVH) and LV outflow tract obstruction (LVOTO). The role of surgical myectomy in such patients is unknown. We sought to compare clinical features and outcomes of patients with NS and LVOTO with age- and gender-matched patients with nonsyndromic, obstructive hypertrophic cardiomyopathy (HC) after myectomy. Two cohorts were selected and retrospectively analyzed using Mayo Clinic databases from 1996 to 2014. Subjects included patients with NS with LVH and LVOTO and nonsyndromic controls with obstructive HC. Twenty-three patients with NS and LVH were identified, of whom 12 (8 males) underwent myectomy (10 septal and 2 combined septal/apical) for severe LVOTO (10 pediatric and 2 adults; 13 ± 10 year old [range 1 to 39]). Similar echocardiographic improvements were noted in both groups. There were no perioperative deaths. Residual gradients were slightly higher in patients with NS. No improvement was noted in left atrial volume after myectomy in patients with NS. At early follow-up, the majority showed improvement in the New York Heart Association class (88% in NS vs 82% in HC, median of 6 and 2 months, respectively). At late follow-up (median of 7 years), the survival rate was 92% in NS and 100% in HC. In patients with NS with LVH and symptomatic LVOTO, myectomy reduces both gradient and the New York Heart Association class, similar to patients with nonsyndromic obstructive HC. Residual gradients were slightly higher, and left atrial dilation persisted in patients with NS. In conclusion, myectomy should be considered in patients older than 1 year with NS and symptomatic LVOTO.
Approximately 20% to 30% of patients with Noonan syndrome (NS) have asymmetric left ventricular hypertrophy (LVH) and left ventricular outflow tract obstruction (LVOTO). Surgical myectomy is considered the primary treatment strategy for relief of drug-refractory or severe LVOTO in patients with obstructive hypertrophic cardiomyopathy (HC). The role of surgical myectomy in resolution of LVOTO and improved long-term survival is well established in these nonsyndromic patients with obstructive HC. The role of myectomy in patients with NS and symptomatic LVOTO that survive the first year of life in this surgical era is unknown. The literature is limited, with only a single case reported, a 23-month-old male with NS who underwent surgical myectomy with early postoperative death. The purpose of this study was to compare clinical features and outcomes of patients with NS and LVOTO with age, gender, and body surface area–matched patients with nonsyndromic, obstructive HC before and after ventricular septal myectomy.
Methods
The Mayo Clinic echocardiographic and surgery database was queried to identify patients with NS who underwent surgical myectomy for LVOTO, who were evaluated at the Mayo Clinic from 1996 to 2014. A previous diagnosis of NS was based on their phenotypic and genotypic presentation according to the guidelines established by the American Academy of Pediatrics. Nonsyndromic patients with obstructive HC requiring myectomy were also identified. Matching was performed blinded to clinical data, consecutively, by matching to age and gender, followed by body surface area within 0.3 m 2 . Mayo Clinic Institutional Review Board approval was obtained before data collection, and informed consent was obtained by patients (or guardian) to participate in clinical research.
Preoperative and postoperative transthoracic echocardiography (TTE) was performed using standard equipment in routine clinical practice. Myocardial wall thickness and cardiac dimensions were obtained using previously described methods. Left ventricular ejection fraction was determined with Simpson’s biplane method of disks. Maximal instantaneous gradient and continuous wave Doppler measurement of LVOT gradient was obtained at rest and during provocation with amyl nitrate or valsalva in patients with gradient at rest <30 mm Hg. The presence of systolic anterior motion (SAM) of mitral valve leaflets was recorded. Mitral valve regurgitation (MR) was identified with color wave Doppler by visual estimation of the MR jet and graded as trivial, mild, moderate, and severe. The septal hypertrophy pattern of HC was delineated from the apical 4 chamber and classified as reverse curve, neutral, sigmoid, and apical subtypes.
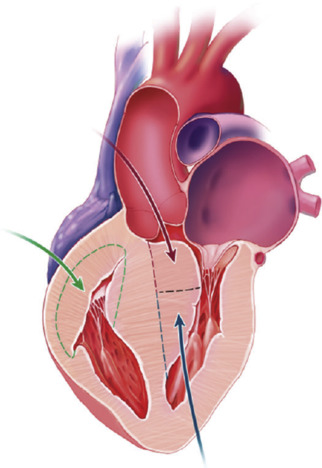
Extracorporeal circulation was instituted through aortic and bicaval or right atrial cannulation with aortic occlusion and cold blood cardioplegia. Patients underwent transaortic extended septal myectomy and/or limited transapical ventriculotomy when the aortic annulus was small or if additional septal resection was required. Accessory papillary muscle bundles and their fibrous attachments were resected additionally to eliminate LVOTO; care was taken to retain all chordal support to the leading edges of the anterior and posterior mitral leaflets. Patients with right ventricular outflow tract (RVOT) obstruction underwent free wall resection through right ventriculotomy and placement of an RVOT patch ( Figure 1 ). Septal resection on the right side was limited to avoid disruption of the many chordal attachments to the tricuspid valve. Augmentation of the RVOT was optimized by free wall resection and RVOT patch placement. Surgical details were recorded including surgical approach, cardiopulmonary bypass time, aortic cross-clamp time, and presystolic and postsystolic pressure LVOT gradients. LVOT peak-to-peak systolic pressure gradients were determined with direct intraoperative measurement by placing needles connected to pressure manometers into the aorta and the LV through the RV and septum and simultaneously recording LV and aortic pressure tracings to determine the difference in pressure. This was undertaken just before myectomy before going on bypass and after myectomy (post-bypass) minutes after hemodynamic stabilization. In patients with low gradients at rest (<20 mm Hg), provocation was performed with induction of premature ventricular contractions by mechanical stimulation of the right ventricle or isoproterenol administration. Muscular specimens from myectomy were submitted to the pathology laboratory for gross and histologic examination.
Postoperative hospital course was evaluated using the electronic medical record (EMR) for complications resulting from surgery. Postoperative discharge echocardiographic data were analyzed to compare the surgical outcomes between the NS cases and the obstructive HC controls in comparison with their baseline echocardiographic data.
For follow-up data, the EMR was queried to determine postoperative status of patients at early follow-up after hospital discharge. Follow-up variables included death, surgical re-intervention, and the New York Heart Association (NYHA) class. Late follow-up mortality data were established using the EMR and the Social Security Death Registry.
Histologic examination of myectomy specimens from all patients in both cohorts was performed using a previously described methodology. Tissue was fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections (4 μm) were stained with hematoxylin and eosin and reviewed in a blinded fashion by a cardiovascular pathologist (JJM). Histologic parameters recorded included the following: myocyte hypertrophy, myocyte disarray, myocyte vacuolization, interstitial fibrosis, and endocardial fibrosis. Myocyte hypertrophy, vacuolization, and endocardial fibrosis were graded semiquantitatively: 0 = absent; 1 = mild; 2 = moderate; 3 = severe. Myocyte disarray was classified according to subtype (herringbone, storiform, or mixed) as was interstitial fibrosis (pericellular or replacement).
Continuous variables were reported as the mean ± SD; categorical variables are given as percentages of group totals. Fisher’s exact test was used to analyze ordinal variables, and student’s t test was used for continuous variables. The chi-square test was used to analyze discontinuous variables. Logistic regression analysis was performed to determine correlates of echocardiographic, hemodynamic, and pathologic data. A p value <0.05 was considered statistically significant.
Results
A total of 5,676 patients with asymmetric LVH were evaluated in Mayo Clinic’s cardiomyopathy clinic during the study time frame. Of these, 29 patients with concomitant syndromes were identified including 23 patients with NS. Twelve of these 23 patients with NS underwent surgical myectomy for severe LVOTO. Blinded to surgical outcomes, we identified 24 age, gender, and body surface area–matched nonsyndromic patients with obstructive HC who had myectomy performed during the same time frame to serve as the control group. These patients were assigned to the control group ( Figure 2 ).
Baseline preoperative clinical and demographic data are listed in Table 1 . Dyspnea was the most common presenting symptom in both groups, whereas diaphoresis was more common in patients with NS. There were no differences in all other symptoms, arrhythmias, or NYHA class. One NS patient had a previous dual-chamber pacemaker placed for recurrent syncope. No significant differences were noted in preoperative TTE findings between the patients with NS and those with obstructive HC ( Table 2 ). All patients in the NS cohort had reverse curvature pattern of septal hypertrophy.
| Variable | Noonan (n = 12) | Controls (n = 24) | p |
|---|---|---|---|
| Age (years) | 13 ± 10 | 13 ± 4 | ns |
| Male | 8/12 (66%) | 16/24 (67%) | ns |
| BSA (m 2 ) | 1.1 ± 0.4 | 1.4 ± 0.4 | ns |
| Syncope | 3/12 (25%) | 7/24 (29%) | ns |
| Diaphoresis | 4/12 (33%) | 0/24 (0%) | 0.01 |
| Angina | 2/12 (17%) | 3/24 (13%) | ns |
| Fatigue | 7/12 (58%) | 21/24 (88%) | 0.09 |
| Dyspnea | 9/12 (75%) | 18/24 (75%) | ns |
| Atrial fib/flutter | 3/12 (25%) | 2/24 (8%) | 0.1 |
| Ventricular tachycardia | 2/12 (17%) | 1/24 (4%) | ns |
| NYHA | |||
| I/II | 6/12 (50%) | 14/24 (58%) | ns |
| III/IV | 6/12 (50%) | 10/24 (42%) | ns |
| Variable | Noonan (n = 12) | Controls (n = 24) | p |
|---|---|---|---|
| Ejection fraction (%) | 72 ± 4 | 72 ± 2 | ns |
| Left ventricular outflow tract maximal instantaneous gradient (mm Hg) | 95 ± 40 | 88 ± 34 | ns |
| Septal wall thickness (mm/m 2 ) | 20 ± 7 | 17 ± 6 | ns |
| Left atrial volume index (ml/m 2 ) | 50 ± 7 | 48 ± 18 | ns |
| Systolic anterior motion (%) | 11/12 (92%) | 22/24 (92%) | ns |
| Pulmonary stenosis (%) | 3/12 (25%) | 2/24 (8%) | ns |
| Mitral regurgitation grade (%) | |||
| None | 0/12 (0%) | 3/24 (13%) | ns |
| Mild | 3/12 (25%) | 8/24 (33%) | ns |
| Moderate | 5/12 (42%) | 10/24 (42%) | ns |
| Severe | 3/12 (33%) | 4/24 (17%) | ns |
Operative data are listed in Table 3 . All patients with NS underwent transaortic extended septal myectomy, whereas 2 underwent combined transaortic and apical septal resection. Mitral valve intervention in the patients without NS was reserved for structural abnormalities of the mitral valve; specifically, no intervention was done on the mitral valve for SAM-mediated MR. Mitral valve intervention was more common in patients with NS because of associated structural abnormalities. Patients with NS had a higher total number of additional procedures including papillary resection, RVOT reconstruction, and mitral valvuloplasty. Compared with the nonsyndromic patients with obstructive HC, patients with NS had a higher intraoperative systolic gradients (p <0.05) and longer cardiopulmonary bypass and aortic cross-clamp times (p <0.05). Postoperative data are displayed in Table 4 . In both groups, postoperative TTE (obtained at 6 ± 3 days in patients with NS and 4 ± 1 day in controls) revealed significant reduction in LVOT gradient, resolution of SAM, and reduction in MR grade. No improvement was noted in left atrial (LA) volume index after myectomy in patients with NS. Residual mean LVOT gradient and LA volume index were higher in patients with NS after surgery. Postoperative hospital duration was similar (7 ± 3 days in patients with NS vs 6 ± 2 days in those with obstructive HC). There were no deaths, extracorporeal membrane oxygenation requirement, heart block, arrhythmias, or new aortic valve insufficiency noted in either group.
| Procedure | Noonan (n = 12) | Controls (n = 24) | p |
|---|---|---|---|
| Ventricular septal myectomy | 12/12 (100%) | 24/24 (100%) | ns |
| Septal & apical myectomy | 2/12 (17%) | 1/24 (4%) | ns |
| Papillary muscle resection | 6/12 (50%) | 6/24 (25%) | ns |
| RVOT reconstruction | 2/12 (17%) | 2/24 (8%) | ns |
| Mitral valvuloplasty | 3/12 (25%) | 0/24 (0%) | 0.03 |
| Pre-bypass intraoperative systolic gradient (mm Hg) | 98 ± 30 | 67 ± 28 | <0.05 |
| Bypass time (minutes) | 66 ± 25 | 50 ± 14 | <0.05 |
| Cross clamp time (minutes) | 50 ± 16 | 39 ± 10 | <0.05 |
| Variable | Noonan (n = 12) | Control (n = 24) | p |
|---|---|---|---|
| LVOT MIG (mmHg) | 29 ± 11 ∗ | 3 ± 3 ∗ | < 0.05 |
| Left atrial volume (ml/m 2 ) | 47 ± 19 | 33 ± 8 ∗ | < 0.05 |
| Systolic anterior motion of the mitral valve resolved | 11/12 (91%) ∗ | 22/24 (92%) ∗ | ns |
| Mitral regurgitation grade improved | 9/12 (66%) ∗ | 16/24 (68%) ∗ | ns |
| Deaths | 0/12 (0%) | 0/24 (0%) | ns |
| ECMO Requirement | 0/12 (0%) | 0/24 (0%) | ns |
| Complete heart block | 0/12 (0%) | 0/24 (0%) | ns |
| Hospitalization (days) | 7 ± 3 | 6 ± 2 | ns |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


