Chapter 39 Surgical Treatment of Abdominal Aortic Aneurysms
Abdominal aortic aneurysms (AAAs) remain a leading cause of death in the elderly. In the United States, ruptured AAAs are the 15th leading cause of death overall and the 10th leading cause of death in men older than age 55.1 In addition, 30% to 40% of patients with ruptured AAAs die after reaching a hospital, but without operation.2 When combined with an operative mortality rate of 40% to 50%,3–7 this results in an overall mortality rate of 80% to 90% for AAA rupture.8–10 Unfortunately, this high mortality rate has not changed over the past 20 years despite improvements in operative technique and perioperative critical care management that have reduced the elective surgical mortality rate to less than 5% in most series.3 Ruptured aneurysms also impose a substantial financial burden on overall healthcare costs. One report estimated that as much as $50 million and 2000 lives could have been saved in 1 year if AAAs had been repaired prior to rupture.11 Another study showed that emergency operations for AAAs resulted in a mean financial loss to the hospital of $24,655 per patient.12 These data have significant implications in an era of healthcare cost containment. For all these reasons, AAAs remain a central focus for vascular surgeons and an important healthcare problem for all physicians.
Definition
Most aortic aneurysms are true aneurysms involving all layers of the aortic wall and are infrarenal in location. As shown by Pierce et al.,13 normal aortic diameter gradually decreases from the thorax (28 mm in men) to the infrarenal location (20 mm in men). At all anatomical levels, normal aortic diameter is approximately 2 mm larger in men than in women and increases with age and increased body surface area.13 Because the average infrarenal aortic diameter is 2 cm, using a 3-cm definition for an infrarenal AAA has been recommended, without the need to consider a more complicated definition based on factors such as gender or body surface area. Although such definitions are useful for large patient groups, in clinical practice with individual patients, defining an aneurysm based on a 50% or greater diameter enlargement compared with the adjacent nonaneurysmal aorta has been recommended.14 This is particularly true for patients with unusually small arteries, in whom even a 2.5-cm local dilation of the infrarenal aorta might be aneurysmal if the adjacent aorta were only 1.5 cm in diameter.
Decision Making for Elective Abdominal Aortic Aneurysm Repair
The choice between observation and elective surgical repair of an AAA for an individual patient at any given point should take into account the (1) rupture risk under observation, (2) operative risk of repair, (3) patient’s life expectancy, and (4) personal preferences of the patient.15,16 Two randomized trials have provided substantial information to assist with this decision-making process. The U.K. Small Aneurysm Trial was the first randomized trial to compare early surgery with surveillance of 4- to 5.5-cm diameter AAAs in 1090 patients aged 60 to 76.17 Those undergoing surveillance underwent repeat ultrasound every 6 months for AAAs 4 to 4.9 in diameter cm, and every 3 months for those 5 to 5.5 cm. If AAA diameter exceeded 5.5 cm, the expansion rate was more than 1 cm/yr, the AAA became tender, or repair of an iliac or thoracic aneurysm was necessary, elective surgical repair was recommended. At the initial report in 1998, after a mean 4.6 years’ follow-up, there was no difference in survival between the two groups. After 3 years, patients who had undergone early surgery had better late survival, but the difference was not significant. It was notable that more than 60% of patients randomized to surveillance eventually underwent surgery at a median time of 2.9 years. Rupture risk among those undergoing careful surveillance was 1% per year.
In 2002, the U.K. trial participants published results of long-term follow-up.18 At 8 years, there was a small survival advantage in the early surgery group (7.2% improved survival). However, the proportion of deaths due to rupture of an unrepaired AAA was low (6%). The early surgery group had a higher rate of smoking cessation, which may have contributed to a reduction in overall mortality. An additional 12% of surveillance patients underwent surgical repair during extended follow-up to bring the total to 74%. Fatal rupture occurred in only 5% of men but 14% of women in the surveillance group. Risk of rupture was more than four times higher for women than men. This prompted participants to recommend a lower-diameter threshold for elective AAA repair in women.
The Aneurysm Detection and Management (ADAM) study conducted at the U.S. Department of Veterans Affairs (VA) hospitals was published in 2002.19 In this trial, 1163 veterans (99% male) aged 50 to 79 with AAAs 4 to 5.4 cm in diameter were randomized to either surveillance or early surgery. Surveillance entailed ultrasound or computed tomography (CT) scan every 6 months, with elective surgery for expansion to 5.5 cm, expansion of greater than 0.7 cm in 6 months or greater than 1 cm in 1 year, or development of symptoms attributable to the AAA. Computed tomography was used for initial evaluation, with AAA diameter defined as the maximal cross-sectional measurement in any plane that was perpendicular to the aorta. Ultrasound was used for the majority of surveillance visits, but CT was used when the diameter reached 5.3 cm. Patients with severe heart or lung disease were excluded, as were those who were not likely to comply with surveillance. As in the U.K. trial, there was no survival difference between the two strategies after a mean follow-up of 4.9 years. Similarly, more than 60% of patients in the surveillance arm underwent repair. Initial AAA diameter predicted subsequent surgical repair in the surveillance group; 27% of those with AAAs initially 4 to 4.4 cm underwent repair during follow-up, compared with 53% of those with 4.5 to 4.9 cm, and 81% of those with 5- to 5.4-cm diameter AAAs. Operative mortality was 2.7% in the early surgery group and 2.1% in the surveillance group. Rupture risk in those undergoing surveillance was 0.6% per year. This trial confirmed the results of the U.K. trial, demonstrating lack of benefit of early surgery for AAAs 4 to 5.5 cm, even if operative mortality is low. Compliance with surveillance was high in both trials. More recently, Ouriel et al. reported results of 728 patients who were randomized to either ultrasound surveillance or early endovascular AAA repair (EVAR). Mean follow-up of 20 ± 12 months demonstrated no difference in AAA rupture, aneurysm-related death, or overall mortality between groups.20
Taken together, these two large randomized studies indicate that it is generally safe to wait for AAA diameter to reach 5.5 cm before performing surgery in selected men who are compliant with surveillance, even if their operative mortality is predicted to be low even in the endovascular era. However, compliance in these carefully monitored trials of selected patients was high. In another VA population, Valentine et al.21 reported that 32 of 101 patients undergoing AAA surveillance were noncompliant despite several appointment reminders, and 3 or 4 of these 32 patients experienced rupture. Additionally, the increased rupture risk for women seen in the U.K. trial highlights the need to individualize treatment on the basis of a careful assessment of individual patient characteristics (rupture risk, operative risk, life expectancy, and patient preferences).
Elective Operative Risk
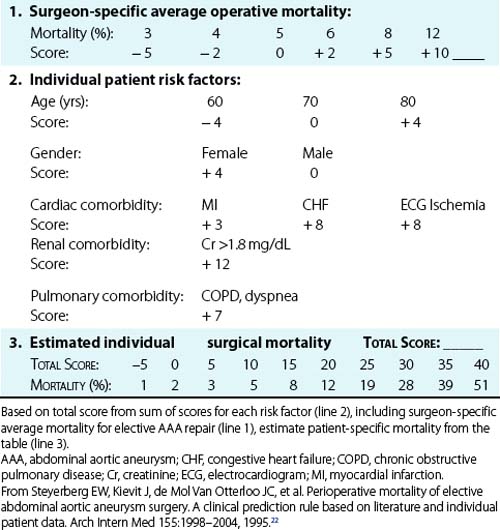
As expected, considerable variation in operative risk occurs among individual patients and depends on specific risk factors. A meta-analysis by Steyerberg et al.22 identified seven prognostic factors that were independently predictive of operative mortality with elective AAA repair and calculated the relative risk for these factors (Table 39-1). The most important risk factors for increased operative mortality were renal dysfunction (creatinine (Cr) > 1.8 mg/ dL), congestive heart failure (CHF) (cardiogenic pulmonary edema, jugular vein distension, or the presence of a gallop rhythm), and ischemic changes on resting electrocardiogram (ECG; ST depression >2 mm). Age had a limited effect on mortality when corrected for the highly associated comorbidities of cardiac, renal, and pulmonary dysfunction (mortality increased only 1.5-fold per decade). This explains the excellent results reported in multiple series in which selected octogenarians have undergone elective AAA repair, with mortality comparable to younger patients.23
Table 39-1 Independent Risk Factors for Operative Mortality After Elective Abdominal Aortic Aneurysm Repair
| Risk Factor | Odds Ratio* | 95% Confidence Interval |
|---|---|---|
| Cr > 1.8 mg/dL | 3.3 | 1.5-7.5 |
| CHF | 2.3 | 1.1-5.2 |
| ECG ischemia | 2.2 | 1-5.1 |
| Pulmonary dysfunction | 1.9 | 1-3.8 |
| Older age (per decade) | 1.5 | 1.2-1.8 |
| Female gender | 1.5 | 0.7-3 |
CHF, congestive heart failure; Cr, creatinine; ECG, electrocardiogram.
* Indicates relative risk compared with patients without that risk factor.
From Steyerberg EW, Kievit J, de Mol Van Otterloo JC, et al: Perioperative mortality of elective abdominal aortic aneurysm surgery. A clinical prediction rule based on literature and individual patient data. Arch Intern Med 155:1998–2004, 1995.22
On the basis of their analysis, Steyerberg et al.22 developed a clinical prediction rule to estimate the operative mortality for individual patients undergoing elective AAA repair (Table 39-2). This scoring system takes into account the seven independent risk factors plus the average overall elective mortality for a specific center. To demonstrate the impact of the risk factors on a hypothetical patient, it can be seen that the predicted operative mortality for a 70-year-old man in a center with an average operative mortality of 5% could range from 2% if no risk factors were present to more than 40% if cardiac, renal, and pulmonary comorbidities were all present. Obviously this would have a substantial impact on the decision to perform elective AAA repair. A similar Bayesian model for perioperative cardiac risk assessment in vascular patients has been reported by L’Italien et al.,24 which demonstrated the added predictive value of dipyridamole-thallium studies in patients with intermediate risk for cardiac death. This study also demonstrated the protective effect of coronary artery bypass surgery within the previous 5 years, which reduced the risk of myocardial infarction (MI) or death following AAA repair by 2.2-fold. Although this type of statistical modeling cannot substitute for experienced clinical judgment, it helps identify high-risk patients who might benefit from further evaluation, risk factor reduction, or medical management instead of surgery if AAA rupture risk is not high.
The review of Hallin et al.5 supports the findings of Steyerberg’s group that renal failure is the strongest predictor of mortality, with a four- to ninefold increased mortality risk. Cardiac disease (a history of either coronary artery disease [CAD], CHF, or prior MI) was associated with a 2.6- to 5.3-fold greater operative mortality risk. Older age and female gender appeared to be associated with increased risk, but the evidence was not as strong. Valuable data regarding predictors of operative risk have been generated by prospective trials. In the Canadian Aneurysm Study, overall operative mortality was 4.8%.25 Preoperative predictors of death were ECG evidence of ischemia, chronic pulmonary disease, and renal insufficiency. The randomized U.K. Small Aneurysm Trial found older age, lower forced expiratory volume in 1 second (FEV1), and higher Cr to be associated with mortality on univariate analysis.26 With multivariate analysis, the effect of age was diminished, whereas renal disease and pulmonary disease remained strong predictors of operative mortality. The predicted mortality ranged from 2.7% for younger patients with below average Cr and above average FEV1 to 7.8% in older patients with above average Cr and below average FEV1. The U.K. trialists noted that the Steyerberg prediction rule did not work well for the U.K. trial patients. However, they did not gather information on a history of CHF (one of the strongest predictors in Steyerberg’s analysis) in the randomized trial. Female gender has also been found to be associated with higher operative risk in several population-based studies using administrative data.3,22,27,28 However, these databases may suffer from inaccurate coding of comorbidities and thereby lack of ability to fully adjust for comorbid conditions.29 Gender has not been found to be associated with operative mortality in prospective trials.26,30
More recently, a study by Beck et al. from the Vascular Study Group of New England assessed risk factors associated with 1-year mortality following open AAA repair and EVAR. In this study, 1387 consecutive patients between 2003 and 2007 underwent elective AAA repair, including 748 who underwent open repair and 639 who underwent EVAR. Consistent with other studies, factors associated independently with 1-year mortality following open AAA repair included age (>70 years), chronic obstructive pulmonary disease (COPD), chronic renal insufficiency (Cr >1.8 mg/dL) and suprarenal aortic clamp site. Likewise, factors associated with 1-year mortality following EVAR included CHF and AAA diameter. One-year mortality correlated linearly with the number of risk factors present, and accordingly should be factored into decision making when considering elective AAA repair.31
Life Expectancy
Assessment of life expectancy is crucial to determine whether an individual patient will benefit from prophylactic repair of an AAA. Many patients with AAAs have been long-term smokers. Most AAA patients also have extensive comorbid disease, particularly CAD, COPD, hypertension, hyperlipidemia, cerebrovascular disease, and cancer.32–37 Many of these chronic conditions increase operative risk, as noted earlier. In addition, these factors impact life expectancy. Patients who survive elective AAA repair have a reduced life expectancy compared to age- and gender-matched populations.38–40 In 2001, Norman et al.41 reviewed 32 publications over 20 years that described long-term survival after AAA repair. They found that the mean 5-year survival after AAA repair was 70%, compared with 80% in the age- and gender-matched population without AAA. Predictors of late death after successful AAA repair include age, cardiac disease, chronic pulmonary disease, renal insufficiency, and continued smoking.38,42,43 The U.K. trialists found (after adjustment for age, gender, and AAA diameter but not cardiac disease) that both FEV1 and current smoking status (plasma cotinine) predicted late death.43
Surgical Decision Making
In patients with symptomatic AAAs, operative repair is nearly always appropriate because of the high mortality associated with rupture or thrombosis and the high likelihood of limb loss associated with peripheral embolism. Occasionally, high-risk patients or those with short life expectancies may choose to forego emergency repair of symptomatic AAAs, but in general, surgical decision making for symptomatic AAAs is straightforward. A contemporary analysis of outcomes of symptomatic AAAs by De Martino et al. from the Vascular Study Group of New England recently assessed 2386 AAA repairs in whom 1959 were elective, 156 were symptomatic, and 271 were ruptured. EVAR was successfully performed in 945 elective patients, 60 symptomatic patients, and 33 ruptured AAA patients, respectively. Hospital mortality was 1.7% for elective AAA, compared to 1.3% for the symptomatic cohort. One- and 4-year survival was determined to be 83% and 68%, respectively, among the symptomatic group, which compared favorably to the elective group with 89% and 73% 1- and 4-year survival.44
For those with asymptomatic AAAs, randomized trials have provided assurance that the typical male patient can generally be safely monitored with careful ultrasound surveillance until the AAA reaches 5.5 cm, at which time elective repair can be performed. However, decision analyses and cost-effectiveness modeling have previously demonstrated that individual patient rupture risk, operative risk, and life expectancy have to be considered to determine the optimal threshold for intervention.15,16,45,46 Both the U.K. and ADAM trials excluded patients who were considered “unfit” for repair, highlighting the fact that those with high operative risk and short life expectancy should have a threshold diameter greater than 5.5 cm. In the U.K. trial, the rupture risk for women was 4.5-fold higher than for men, prompting the authors to recommend a lower threshold for women than men, so it seems logical to consider other factors that may make rupture more likely during surveillance as well. In both randomized trials, 60% to 75% of patients undergoing surveillance eventually underwent AAA repair.19,47 In the U.K. trial, 81% of those with initial diameters 5 to 5.4 cm eventually underwent repair. Clearly, for many patients with this size AAA, the question is not whether to perform AAA repair but when. Therefore, in patients with AAA diameters approaching 5.5 cm whose life expectancy is expected to be more than 5 years and whose operative risk is estimated to be low, the patient should be informed that AAA repair would likely be required within the next few years. This subgroup of patients could be offered surgery at a time when it is convenient for them, with the understanding that waiting for expansion to 5.5 cm has little risk. In these cases, patient preference should weigh heavily in the decision-making process. For those with multiple risk factors for rupture, long life expectancy, and low operative risk, it would seem prudent to recommend AAA repair at less than 5.5 cm. Additionally, the ability of the patient to comply with careful surveillance should be considered. Although the recent randomized trials have provided a great deal of information to guide decision making, clinicians should not adopt a one-size-fits-all policy for treating patients with AAA. Moreover, with a progressively aging population in mind, quality-of-life assessments should likely be factored into decision-making analyses as well.
Preoperative Assessment
Patient Evaluation
A careful history, physical examination, and basic laboratory data are the most important factors for estimating perioperative risk and subsequent life expectancy. These factors may not only influence the decision to perform elective AAA repair, but they may focus preoperative management to reduce modifiable risk. Assessments of activity level, stamina, and stability of health are important and can be translated into metabolic equivalents to help assess both cardiac and pulmonary risks.48 Because COPD is an independent predictor of operative mortality,26,30 it should be assessed by pulmonary function studies as well as room air arterial blood gas measurement in patients who have apparent pulmonary disease. In some cases, preoperative treatment with bronchodilators and pulmonary toilet can reduce operative risk.49 In more extreme cases, pulmonary risk may substantially reduce life expectancy, and in these patients, formal pulmonary consultation may be helpful to estimate survival. Serum Cr is one of the most important predictors of operative mortality25 and must be assessed. The impact of other diseases such as malignancy on expected survival should also be carefully considered.
It is well established that patients with AAAs have a high prevalence of CAD. By performing routine preoperative coronary arteriography at the Cleveland Clinic in 1979, Hertzer et al.50 reported that only 6% of patients with AAAs had normal arteries; 29% had mild to moderate CAD, 29% had advanced compensated CAD, 31% had severe correctable CAD, and 5% had severe uncorrectable CAD. Furthermore, this study established that clinical prediction of the severity of CAD was imperfect because 18% of patients without clinically apparent CAD had severe correctable CAD on arteriography, compared with 44% of patients whose CAD was clinically apparent. This pivotal study has led to intense efforts to identify risk factors and algorithms that more accurately predict the presence of severe CAD that would justify its correction before AAA repair, or would lead to avoiding AAA repair. A number of clinical parameters such as angina, history of MI, Q-wave on ECG, ventricular arrhythmia, CHF, diabetes, and increasing age have been reported to increase the risk of postoperative cardiac events.51 Various combinations of these risk factors have been used to generate prediction algorithms for perioperative cardiac morbidity.48 In general, these algorithms identify low-risk, high-risk, or intermediate-risk patients. For high-risk patients, such as those with unstable angina, more sophisticated cardiac evaluation is required, whereas low-risk patients may undergo elective AAA repair without further testing. For intermediate-risk patients, who comprise the vast majority with AAAs, decision making is more difficult and may be assisted by additional cardiac testing.51
Aneurysm Evaluation
Most surgeons recommend a preoperative imaging study using CT scanning, magnetic resonance imaging or angiography (MRI/MRA), or arteriography. Contrast-enhanced CT appears to be the most useful study for preoperative AAA evaluation when considering information obtained, invasiveness, and cost (also see Chapter 14). This is particularly true for spiral CT scanning, with thin “slices” in the region of interest. This allows not only accurate size measurements but also accurate definition of the relationship of an AAA to visceral and renal arteries. Furthermore, CT scanning aids in identifying venous anatomical anomalies (e.g., retroaortic left renal vein, duplicated vena cava) or renal abnormalities (e.g., horseshoe or pelvic kidney) that would influence operative techniques and approach. Computed tomography is the technique of choice to identify suspected inflammatory aneurysms and may reveal unsuspected abdominal pathology such as associated malignancy or gallbladder disease. In centers with experience with these techniques, CT angiography has made percutaneous intraarterial angiography unnecessary in the vast majority of AAA patients. Moreover, in the EVAR era, CT is vital for case planning and accurate detailed anatomical assessment of aortic neck anatomy, iliac artery anatomy and tortuosity, and perirenal mural thrombus burden among other factors. In addition, three-dimensional (ED) modeling of contemporary CT scanning is useful prior to EVAR as well as open AAA repair and has largely supplanted the role of conventional angiography.
Magnetic resonance imaging is comparable with CT in terms of AAA measurement accuracy and other preoperative planning issues (also see Chapter 13). It avoids intravenous contrast, which may represent an advantage over CT for some patients. Because it is more expensive and time consuming, it also is not as widely used as CT. When MRA is included with this technique, however, it can significantly increase the value in patients where additional imaging would otherwise be required.
Surgical Treatment
For the past 40 years, AAAs have been repaired using the technique of endoaneurysmorrhaphy with intraluminal graft placement, as described by Creech.52 This procedure is described later in the section on transperitoneal approach. Development of this technique was based in part on the failure of previous “nonresective” operations now of only historical interest, including aneurysm ligation, wrapping, and attempts at inducing aneurysm thrombosis that yielded uniformly dismal results. Abdominal aortic aneurysm thrombosis by iliac ligation combined with axillobifemoral bypass enjoyed a brief resurgence in popularity for high-risk patients but demonstrated a high complication rate, including late aneurysm rupture, and an operative mortality rate comparable with conventional repair in similar patients.53–57 Thus this technique was similarly abandoned. As an alternative to standard open AAA repair, Shah and Leather et al.58 proposed exclusion of an AAA with bypass to reduce operative blood loss. However, this group has recently published long-term follow-up and no longer recommends this procedure owing to persistent flow in the excluded AAA sac and rupture in rare cases.59 In another attempt to reduce the invasiveness of open AAA repair, the use of laparoscopy as an adjunct has been suggested to assist AAA repair. This approach uses laparoscopic techniques to dissect the aneurysm neck and iliac arteries, followed by a standard endoaneurysmorrhaphy through a mini-laparotomy. Cohen et al.60 have reported their results in 20 patients to demonstrate the feasibility of this approach, but a clear benefit has not been shown; intraoperative, intensive care unit (ICU), and total hospital duration were comparable with conventional AAA repair. Further experience with this technique may identify a subgroup of patients for whom a laparoscopic-assisted AAA repair is advantageous.
EVAR (see Chapter 40) repair was introduced by Parodi in 1991 and has rapidly gained in popularity in the United States after reports of clinical trials and subsequent U.S. Food and Drug Administration (FDA) approval.61 Endovascular AAA repair has been shown to reduce operative morbidity, mortality, length of stay, and disability compared with open repair.62–65 Recovery time is shorter after endovascular repair than open repair,63,66 but endovascular repair may not be as durable.67–74 Frequent and lifelong surveillance is required after endovascular repair, along with reintervention or conversion to open repair in some. There appears to be a small ongoing risk of rupture after endografting as well. Decision analysis suggests that there is little difference in outcome between open and endovascular repair for most patients.72 However, endovascular AAA repair is usually recommended for those with good anatomy for EVAR or those with marginal anatomy but high operative risk for open surgery. Open surgery may be preferred for younger, healthier patients in whom there is little difference in operative risk between the two strategies, and for whom long-term durability is a concern, although contemporary stent grafts appear to have improved durability from their initial constructs and are now recommended for most patients with acceptable anatomy (see Chapter 40).
To date, there are several important randomized trials comparing open AAA repair with endovascular repair. Specifically, in the EVAR I and DREAM trials, patients were randomized to either open repair or EVAR. The EVAR I study demonstrated a 3% lower initial mortality associated with endovascular treatment, with a persistent associated reduction in AAA-related death at 4 years. However, there was no overall improvement in all-cause mortality between groups. Likewise, the DREAM trial demonstrated an operative mortality advantage associated with EVAR compared to open surgical repair, but 1-year survival was similar between groups. The EVAR II study randomized patients unfit for open AAA repair to either EVAR or no surgical therapy. This trial failed to demonstrate a survival advantage for the EVAR treatment group compared to the no treatment group. It should be noted, however, that most ruptures in the EVAR group occurred during a prolonged delay before surgery, making the results in this group appear worse. In addition, 27% of patients in EVAR II crossed over from the no treatment group to the EVAR group, potentially limiting the study’s findings.71,75–77 Likewise, the VA Open vs. Endovascular AAA repair (OVER) study randomized patients to either open AAA repair or EVAR. Results demonstrated diminished perioperative mortality in the EVAR group compared to the open repair group (0.5% vs. 3.0%). However, there was no observed difference in mortality at 2 years between groups. This study also demonstrated diminished median procedure times, blood loss, transfusion requirement, duration of mechanical ventilation, hospital length of stay, and ICU length of stay in the EVAR group.78
Perioperative Management
Preoperative intravenous antibiotics are administered to reduce the risk of prosthetic graft infection.79 Ample intravenous access, intraarterial pressure recording, and Foley catheter monitoring of urine output are routine. For patients with significant cardiac disease, pulmonary artery catheters are frequently used to guide volume replacement and vasodilator or inotropic drug therapy, both intraoperatively and in the early postoperative period. Mixed venous oxygen tension measurement, available with these catheters, can provide an additional estimate of global circulatory function. Transesophageal echocardiography (TEE) can be useful in certain patients to monitor ventricular volume and cardiac wall motion abnormalities and to guide fluid administration and use of vasoactive drugs. Despite the frequent use of pulmonary artery catheters, studies examining their use during AAA surgery have not demonstrated added value.80,81 However, these studies have usually excluded high-risk patients who are most likely to benefit from such monitoring. These techniques are not without risk, so selective use is probably more appropriate than routine application.
The volume of blood lost during AAA repair often requires blood replacement. Therefore, intraoperative autotransfusion as well as preoperative autologous blood donation has become popular, primarily to avoid the infection risk associated with allogeneic transfusion. Studies of the cost-effectiveness of such procedures, however, question their routine use.82–84 Autologous blood donation is less important for elderly patients in whom life expectancy is shorter than the usual time for development of transfusion-associated viral illness. Autologous blood donation does not appear to be cost-effective in elderly cardiovascular patients, because the allogenic blood pool has become safer and the transfusion requirement for elective AAA repairs lower.82 Intraoperative autotransfusion during AAA repair is widely used because of the documented safety of this technique.85 Because it is usually difficult to predict the volume of blood loss during AAA repair, most surgeons employ autotransfusion in case blood loss becomes extensive. Optimizing oxygen delivery to patients with reduced cardiac output by maintaining an adequate hematocrit appears beneficial in patients undergoing AAA repair. One study has shown that a postoperative hematocrit of less than 28% was associated with significant cardiac morbidity in vascular surgery patients.86
Maintenance of normal body temperature during aortic surgery is important to prevent coagulopathy, allow extubation, and maintain normal metabolic function. In a review of patients undergoing elective AAA repair, Bush et al.87 noted significantly more organ dysfunction (53% vs. 29%) and higher mortality (12% vs. 1.5%) in hypothermic patients (temperature <34.5 °C) compared with normothermic patients. The only predictor of intraoperative hypothermia was female gender, whereas prolonged hypothermia was related to initial hypothermia, indicating the difficulty in rewarming cold patients. A recent randomized trial found significantly reduced cardiac morbidity (1.4% vs. 6.3%) in patients who were normothermic (36.7 °C) rather than hypothermic (35.4 °C) intraoperatively.88 To prevent hypothermia, a recirculating warm forced-air blanket should be placed in contact with the patient, and intravenous fluids, including any blood returned from an autotransfusion device, should be warmed before administration.
The role of ischemic preconditioning in lowering the incidence of perioperative MI during open AAA repair remains undefined, although there are data to support its potential benefit. In the largest study to date, Ali et al. randomized 82 patients undergoing elective open AAA repair to receive remote ischemic preconditioning or not. The technique involves sequential clamping of each common iliac artery (CIA) for 10 minutes, followed by 10 minutes of respective reperfusion. The authors demonstrated that patients undergoing remote ischemic preconditioning had both diminished rates of postoperative MI and diminished critical care length of stay compared to the control groups.89
Anesthesia
Nearly all patients undergo general anesthesia for AAA repair. Supplemental use of continuous epidural anesthesia, begun immediately preoperatively and continued for postoperative pain control, is increasing in popularity.90 This technique allows a lighter level of general anesthesia to be maintained while controlling pain through the epidural blockade. Additional benefits may include a reduction in the sympathetic catecholamine stress response, which might decrease cardiac complications. One randomized trial comparing general anesthesia with combined general and epidural anesthesia demonstrated decreased deaths, cardiac events, infection, and overall complications.91 These benefits, however, were not observed in another randomized trial,92 suggesting that the details of perioperative management and patient selection may determine the impact of epidural anesthesia. Furthermore, it is possible that the major benefit of epidural anesthesia accrues in the postoperative period rather than intraoperatively.93
Perioperative β-adrenergic blockade remains somewhat more controversial, given recent findings of randomized controlled trials.94 Earlier studies by Pasternack et al.95 demonstrated that patients who underwent vascular surgery and received metoprolol immediately before operation had significantly lower heart rates and less intraoperative myocardial ischemia than untreated controls. Mangano et al.96 performed the first randomized placebo-controlled trial to assess the effect of atenolol (given intravenously immediately before and after surgery and orally during that hospitalization) in patients at risk for CAD who underwent noncardiac surgery. A significant reduction in mortality extending 2 years after discharge was observed in the atenolol-treated patients (3% vs. 14% 1-year mortality) because of reduction in death from cardiac causes. In a separate analysis, they noted that atenolol-treated patients had a 50% lower incidence of myocardial ischemia during the first 48 hours after surgery and a 40% lower incidence during postoperative days 0 to 7.97 Patients with perioperative myocardial ischemia were significantly more likely to die within 2 years after surgery. Poldermans et al.98 performed a randomized trial of perioperative β-blockade with bisoprolol in patients with abnormal dobutamine echocardiograms undergoing aortic or lower-extremity arterial reconstruction. They found that perioperative cardiac death was significantly reduced from 17% (placebo) to 3% (bisoprolol). Additionally, nonfatal MI occurred in 17% of those given placebo but in none of those given bisoprolol. A subsequent publication from the same authors demonstrated that during a mean follow-up of 22 months, cardiac events were significantly lower in those who had received perioperative β-blockade (12% vs. 32%).99
More recently, however, results from the POISE trial, a randomized controlled trial reflecting 190 hospitals, 23 countries, and an enrollment of 8351 patients, provided different results. This study compared the effects of perioperative extended release metoprolol succinate with a limited titration scheme to placebo among patients undergoing noncardiac surgery. Results demonstrated that there was a significant reduction in the composite endpoint of cardiovascular death, nonfatal MI, and nonfatal cardiac arrest among patients receiving perioperative β-blocker therapy. However, the study also revealed that there were more deaths and strokes among the treated group compared to placebo.94 Although these findings seemingly conflict, perioperative β-blocker use is valuable when titrated to heart rate, but not when applied at initial high dose or without respect to the patient’s hemodynamics.100
Given this knowledge, it has been suggested that β-blockers are underused, likely because of fears about use in patients with COPD or prior heart failure. However, chronic β-blocker usage is now known to improve outcomes in patients with heart failure.101,102 Additionally, Gottlieb et al.101 demonstrated that COPD should not be considered a contraindication for β-blockade. They found a 40% reduction in risk of death after MI in patients with COPD who were taking β-blockers compared with those who were not. In Mangano’s trial, the only exclusion criteria were preexisting ECG abnormalities that would preclude detection of new ischemic events. β-Blockers were withheld during the trial only for a heart rate of less than 55 beats/min, systolic blood pressure less than 100 mmHg, acute bronchospasm, current evidence of CHF, or third-degree heart block. The weight of evidence supports routine use of β-blockers for nearly all patients undergoing AAA repair.
Antiplatelet use remains common in this patient cohort, concordant with American College of Cardiology/American Heart Association (ACC/AHA) guidelines for noncardiac surgery. Associated bleeding risk with such agents, including aspirin and clopidogrel, remains controversial. In a recent study by the Vascular Study Group of New England, however, preoperative antiplatelet use (aspirin alone, clopidogrel alone, combined dual therapy) was not significantly associated with increased serious bleeding complications, measured as reoperation for bleeding across a spectrum of commonly performed vascular procedures including EVAR, open AAA repair, carotid endarterectomy, and lower-extremity bypass.103
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree