Surgical Management for Chronic Venous Insufficiency
Mark D. Iafrati
Thomas F. O’Donnell Jr.
Diagnostic Considerations
Chronic venous insufficiency (CVI) represents advanced clinical sequelae of prolonged or refractory venous disease in which lower-extremity swelling, pain, pigmentary changes, and ulceration may be present. Previous chapters in this book have described the natural history (see Chapter 65), as well as the evaluation and nonoperative management (see Chapter 72) of chronic venous insufficiency. The time-tested tenets of elevation, compression, exercise, and skin care are fundamental to a comprehensive approach to the management of CVI; however, to be effective, these interventions require a high degree of patient compliance. For many patients with active lifestyles or physically demanding employment, elevation is not practical. Impediments to the use of compression garments include hot and humid environments, limited hand strength (arthritis), poor flexibility (can’t reach their feet), and the cost of stockings, which frequently is not covered by insurers. Unfortunately, even when recommendations for appropriate conservative therapy are adhered to, the underlying venous pathology remains, and some patients will require surgical treatment to potentially obtain adequate symptom relief or ulcer healing.
Surgical interventions for CVI are designed to correct the hemodynamic perturbations in the deep, superficial, and perforating venous systems of the leg. Appropriate surgical decision making in CVI requires a thorough understanding of the clinical status of the limb, the etiology of the pathologic process, the anatomic distribution of disease in various veins segments, and the pathologic process (reflux or obstruction). The CEAP classification system, adopted by the Society for Vascular Surgery and the American Venous Forum, provides a useful framework for organizing and reporting this information.
Pathogenesis
Both venous obstruction and valvular reflux are known to result in CVI. Superficial, deep, or perforator vein disease alone or in combination can result in all of the sequelae of CVI.
Secondary Valvular Incompetence
Venous thrombosis that initially results in obstruction to flow recanalizes in 80% of cases, especially when distal veins are involved, resulting in valvular reflux. Obstruction alone accounts for less than 5% of symptomatic deep venous pathology. Indeed, it has been our experience that the typical findings of advanced CVI—pigmentation, lipodermatosclerosis, and skin breakdown—are unusual with obstruction alone. Patients with pure iliac vein obstruction usually develop claudication and/or edema without the marked skin changes, unless valvular incompetence is present. There is a close association between venous reflux and clinical venous disease; however, it is clear that not all reflux results in varicose veins and not all clinical venous disease is accompanied by reflux. Although the relationship between venous hemodynamics and clinical symptoms is far from absolute, these relationships are nevertheless useful in understanding the pathophysiology of venous disease and are relied upon for clinical decision making.
Primary Valvular Incompetence
Recent data suggest that intrinsic vein wall abnormalities lead to dilation with subsequent valvular insufficiency. Compared with normal veins, varicose veins show increased diameter of the lumen and hypertrophy of the wall, mainly the intima, due to increased collagen fibers. Collagen fibers also lose their normal pattern and show abnormal forms. Elastic fibers lose their regular laminar arrangement and form clumps or scattered fragments. The distribution of wall degeneration in varicose veins is not uniform. Some segments may be thickened and fibrotic while others are aneurysmal. These structural changes in the vessel wall account for much of the loss of physiologic function of the vein.
While the exact triggers and mechanisms that lead to compromised vein walls and valves remain unclear, an inflammatory process may be an early participant. Indicators of inflammatory processes include elevation of endothelial permeability; attachment of circulating leukocytes to the endothelium; infiltration of monocytes, lymphocytes, and mast cells into the connective tissue; and development of fibrotic tissue infiltrates and several molecular markers, such as growth factor or membrane adhesion molecule generation.
Indications and Contraindications
The indications for surgical intervention in chronic venous insufficiency run the gamut
from purely cosmetic considerations in the treatment of telangiectasias to limb salvage in refractory venous stasis ulcers. Acknowledging the potential cosmetic benefits of surgical interventions, this chapter will address only interventions aimed at the control of signs and symptoms of advanced CVI (swelling, pain, skin changes, and ulceration). Table 73-1 outlines our approach to selecting patients for venous surgery.
from purely cosmetic considerations in the treatment of telangiectasias to limb salvage in refractory venous stasis ulcers. Acknowledging the potential cosmetic benefits of surgical interventions, this chapter will address only interventions aimed at the control of signs and symptoms of advanced CVI (swelling, pain, skin changes, and ulceration). Table 73-1 outlines our approach to selecting patients for venous surgery.
Table 73-1 Criteria for Selecting Patients for Venous Surgery | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Certainly the sine qua non for surgical intervention is the presence of venous disease. No intervention should be undertaken without clear documentation of venous disease. However, in many cases, varicose veins are innocent bystanders in the legs of patients with other diseases that result in painful, swollen, or ulcerated legs; therefore, a firm diagnosis of venous disease as the etiology of the presenting symptoms is required prior to intervening. Venous diseases are often present coincident with other vascular (arterial occlusive disease, lymphedema, arteritides) and nonvascular (congestive heart failure, lupus, renal failure, dermatitis, and so on) diseases, particularly in the elderly. A thorough history and physical examination to determine the presence or absence of the alternative differential diagnoses is mandatory; ancillary testing is performed as indicated. In general, if any of these confounding processes are identified, they should be treated prior to undertaking surgical intervention for CVI. Poor results are to be anticipated if venous surgery is undertaken in the face of untreated arterial occlusion or rheumatologic diseases.
Once a clear diagnosis of venous insufficiency has been established as the primary etiology of the lower-extremity pathology, a trial of conservative therapy is generally indicated. As previously explained (see Chapter 72), compression and elevation are the mainstays of nonoperative therapies. Most patients with symptoms from venous disease will derive benefit from these nonoperative treatments. The response to compressive treatment helps to confirm a venous etiology of the symptoms, and in many patients it will provide sufficient control of symptoms when used on a chronic basis. However, the addition of surgical therapy in these patients can improve long-term outcome. Patients who are compliant with compressive therapies and elevation and derive some benefit from these maneuvers but fail to completely heal, have an ulcer recurrence, or find the therapy unacceptably restricting are particularly good candidates for surgical intervention.
Once venous disease has been determined to be present, symptomatic, and amenable to surgical treatment the final consideration is the fitness of the patient to undergo a surgical procedure. Elderly patients with significant comorbidities may be best served by aggressive nonoperative programs of compression and elevation due to the increased likelihood of peri-operative complications, modest expected improvement in lifestyle/quality of life, and limited expected life expectancy during which these benefits may accrue. On the contrary, younger fit patients with fewer anticipated surgical complications and a longer life expectancy stand to gain more from surgical intervention.
Anatomic Considerations
CVI has been traditionally classified on the basis of anatomy, function, and clinical severity. The anatomic classification of CVI is important because it links the location of CVI with its subsequent clinical management.
The great saphenous vein may be a complete double system or a branching double system between the knee and the foramen ovale, which is of obvious importance for ablative procedures. In the calf a solitary vein is found in only 65% of cases. There is considerable variability in the number and location of branch veins. However, in nearly 90% of limbs, the great saphenous vein at the calf level is anterior dominant.
The small saphenous vein begins posterior to the lateral malleolus and courses cephalad lateral to the Achilles tendon. This vein takes on a midline position lying on the deep fascia at the junction of the lower and middle thirds of the calf. In the upper third of the calf the small saphenous vein penetrates the deep fascia and proceeds into the popliteal space between the heads of the gastrocnemius muscles. In well over one-half of the cases, the small saphenous vein enters the popliteal vein above the level of the knee joint. By contrast, in roughly one-third of limbs the small saphenous vein joins the great saphenous vein or even the deep muscular veins in the upper thigh. Rarely, the small saphenous may merge with the deep veins of the calf or the great saphenous vein in the upper third of the leg.
Perforating Veins
Perforating veins connect the superficial to the deep venous system. Incompetent perforating veins were most commonly observed about 5 to 10 cm above the medial malleolus. In the normal limb, the perforating veins permit the unidirectional flow of blood from the superficial to the deep venous systems through a set of one-way valves. Perforating veins are either direct, permitting the superficial venous system to communicate directly with the main deep veins, or indirect, such that they connect with the deep veins by way of a muscular vein. The direct perforating veins are relatively constant in anatomic location, whereas the indirect perforators are irregularly distributed. There are six groups of perforating veins in the leg, those of the foot, ankle, leg, knee, thigh, and gluteal regions. According to the revised nomenclature, PVs are further described by anatomic location, i.e., medial, lateral, posterior, paratibial, and so on.
The medial leg perforating veins are clinically most significant. Cadaver studies have identified 7 to 20 medial calf perforating veins, with slightly more than half being direct perforators. These perforators connect the posterior accessory great saphenous vein or other tributaries of the saphenous vein directly with the posterior tibial vein. Less
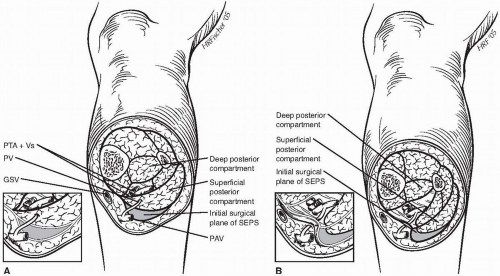
than half of these perforators make direct connections from the great saphenous trunk to the posterior tibial veins, with the majority connecting directly. Although all medial calf perforating veins pass from the deep posterior compartment to the subcutaneous space, only approximately 62% traverse the superficial posterior compartment, as shown in the first panel of Figure 73-1. This anatomic finding has significant surgical implications, because the initial exposure in subfascial endoscopic perforator ligation reveals only the superficial posterior compartment. Identification of the remaining perforating veins requires paratibial fasciotomy.
than half of these perforators make direct connections from the great saphenous trunk to the posterior tibial veins, with the majority connecting directly. Although all medial calf perforating veins pass from the deep posterior compartment to the subcutaneous space, only approximately 62% traverse the superficial posterior compartment, as shown in the first panel of Figure 73-1. This anatomic finding has significant surgical implications, because the initial exposure in subfascial endoscopic perforator ligation reveals only the superficial posterior compartment. Identification of the remaining perforating veins requires paratibial fasciotomy.
In the thigh there are fewer perforating veins; however, they can be clinically very important. The medial thigh and femoral canal PVs communicate between the femoral vein or popliteal vein and the great saphenous vein either directly or indirectly.
The iliac veins are the primary venous outflow for the lower extremities. Venous valves are present in the iliac veins in approximately 27% of cases, being nearly twice as common on the right compared with the left. These valves, when present, can block reflux associated with a Valsalva maneuver and limit the utility of this maneuver in the diagnosis of lower-extremity reflux. Compression of the left iliac vein, as it crosses under the right iliac artery to reach the vena cava, is termed May Thurner syndrome. Iliac vein compression may result in increased resistance to flow and increased venous pressure. In addition to predisposing to venous thrombosis, Raju and Neglen have demonstrated this problem, which may only be visible on intravascular ultrasound (IVUS), to be a common contributor to refractory venous disease.
Finally, the inferior vena cava (IVC), which is the common outflow tract for both legs, is typically right sided, but congenital anomalies, including duplication and transposition, occur in approximately 1% of cases. Because the IVC does not contain valves, it is not implicated in reflux disease but is relevant in venous obstruction when recanalization or bypass is contemplated.
Pre-operative Assessment
While a thorough physical examination reveals a wealth of useful clinical information, vascular imaging techniques can be extremely helpful in the management of venous diseases. Available studies may be broadly divided into physiologic and anatomic examinations, although there is significant overlap in the data. Phlebography and duplex scanning provide detailed anatomic information, which allows for axial and perforator vein mapping, identification of occlusions, and evaluation of segmental vein valve reflux. Physiologic data may be obtained by a variety of plethysmographic techniques. Refer to Chapter 72 for a more detailed discussion of vascular imaging techniques. In brief we find duplex scanning with measurement of segmental valve closure times by the rapid cuff deflation technique to be very useful in selecting patients for surgical intervention. The occasional presence of competent iliac vein valves diminishes the utility of the Valsalva maneuver in assessing lower-extremity reflux. Moreover, the Valsalva maneuver may fail to develop sufficient reversal of venous flow to produce supravalvular pressure changes that cause consistent valve closure. Use of the Valsalva maneuver in the selection of patients for GSV preservation would result in some cases of unrecognized saphenofemoral junction incompetence, which could lead one to inappropriately preserve the GSV with predictable recurrence. Duplex imaging provides detailed anatomic information and is very accurate in the identification of venous thrombosis/occlusion.
Correction of superficial and perforator venous disease is typically undertaken based solely on clinical findings and duplex




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree