53 Surgical Interventions for Congenital Heart Disease
Therapy for congenital heart disease has evolved with surgical and nonsurgical innovations. The development of pediatric cardiac surgery has led to the survival of many children with complex congenital heart disease. These successes have depended on improved diagnoses, advances in surgical technique, and the development of a means for extracorporeal circulation–cardiopulmonary bypass (CPB). Complex repairs for previously fatal lesions such as transposition of the great arteries (TGA) and hypoplastic left-sided heart syndrome (HLHS) have become routine, with declining mortality rates and improved long-term outcomes. The development of transcatheter procedures has made therapeutic cardiac catheterization a viable alternative to surgery for specific congenital cardiac lesions (see Chapter 52).
Surgical Treatment
Palliative Surgical Procedures
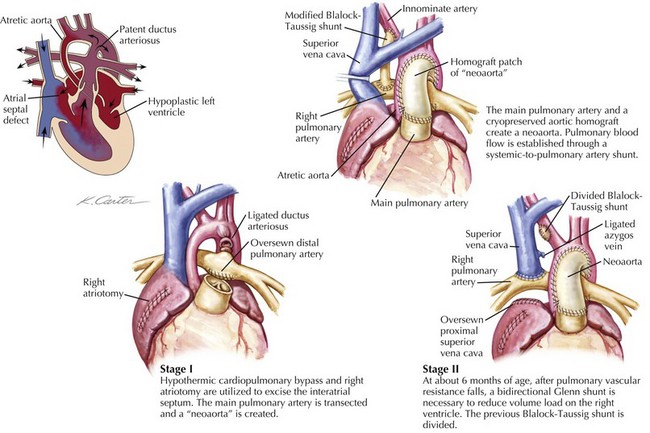
The superior bidirectional cavopulmonary anastomosis, often referred to as the Glenn shunt, is the second stage in the palliation of all or most defects of the single-ventricle type. This shunt involves the disconnection of the superior vena cava (SVC) from the right atrium and a direct anastomosis of the SVC to the right pulmonary artery (Fig. 53-1). This allows all of the systemic venous return from the upper body to flow directly to the lungs. The Glenn shunt is typically performed between 4 and 9 months of age, allowing for enough lung maturity to permit this passive blood flow. Patients will not be fully saturated following the Glenn shunt, since the venous return from the lower body still returns to the heart to mix with the fully oxygenated pulmonary venous blood. Depending on the particular cardiac defect, the procedure may be combined with the removal of a BT shunt or removal of a PA band with division of the main pulmonary trunk from the heart. In either instance, the attendant decrease in the volume load on the heart is beneficial to the function of the single ventricle and its long-term durability.
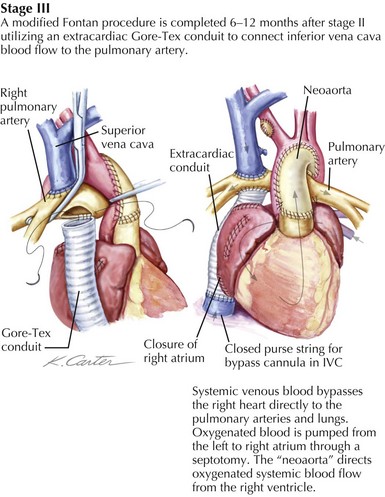
The ability of the pulmonary circulation to accept the entire cardiac output passively is limited and thus the rationale for creating total cavopulmonary circulation in two stages. The Glenn is performed in the first year of life, and the completion of the Fontan is done in the second or third year of life. There are two distinct techniques for completing the Fontan, the lateral tunnel and the extracardiac conduit. The lateral tunnel involves creating a tunnel inside of the right atrium that extends from the opening of the inferior vena cava (IVC) to the opening of the SVC, which is in turn connected to the right pulmonary artery, thus baffling all of the systemic venous return to the lungs. The extracardiac Fontan is created by disconnecting the IVC from the right atrium and sewing a graft of ePTFE from the open end of the IVC to the pulmonary arteries (Fig. 53-2). Using either strategy, a direct connection of the IVC flow to the lungs establishes total cavopulmonary circulation, with the result being that after repair, the patient should have nearly normal oxygen saturation.