Chapter 14 AORTIC VALVE REPLACEMENT/REPAIR Aortic Root Replacement with Composite Valve-Graft (Modified Bentall Procedure) Valve-Sparing Aortic Root Replacement (David and Yacoub Procedures) Aortic Valve Replacement after Previous Coronary Artery Bypass Graft Second Aortic Valve Replacement Procedure Failed Aortic Root Replacement The past decade has shown historic change in the surgical approach to patients with aortic valve disease. During this time, transcatheter aortic valve implantation (TAVI) was developed and tested, leading to a new treatment option approved by the U.S. Food and Drug Administration (FDA) for patients who previously would have been managed by medical therapy or for patients for whom conventional aortic valve replacement (AVR)poses a very high risk (Society of Thoracic Surgeons [STS] predicted risk of mortality [PROM] ≥8).1,2 Additional testing is ongoing in randomized clinical trials to determine the proper use of TAVI in patients for whom AVR poses an intermediate surgical risk (STS score ≥4).3,4 Also, late freedom from structural valve deterioration (SVD) is now available for patients receiving stented bovine pericardial valves and stented porcine valves. The field has been influenced by new valve guidelines regarding the choice of bioprosthetic versus mechanical valve with an emphasis on the patient’s role in decision making as well as expanded indications for treatment of aortic aneurysm in patients with a bicuspid aortic valve (BAV). The impact of these changes has led to the most dramatic change in the clinical practice of aortic valve surgery in decades. Figure 14-1 demonstrates, by year, the changing pattern of valve replacement choices. The graph shows a striking shift in choice of prosthesis. In 2001, 63.6% of aortic valve replacements were bioprostheses, a figure that steadily rose to 81.8% in 2011. The rate of mechanical valve use dropped by more than half, from 30.8% to 14%. The rage of homograft replacement fell from 2.9% to 0.5%, and the Ross procedure has nearly vanished, dropping in rate from 1.0% to 0.1%, a tenfold decrease. FIGURE 14-1 Trend in valve choice in aortic valve replacement in the Society of Thoracic Surgeons National Database. The valve most commonly used to replace the aortic valve is a stented bioprosthetic valve, either bovine pericardium or porcine ( Figures 14-2 and 14-3). The advantages to such a valve are: (1) ease of implantation and the rare occurrence of clinically significant patient prosthesis mismatch due to improving valve hemodynamics, (2) no lifelong need for anticoagulation with warfarin (unless the patient requires it for a different reason), (3) a relatively straightforward future reoperation for SVD, if necessary, and (4) the potential for a valve-in-valve procedure using a transcatheter heart valve for SVD. The most important disadvantage to tissue valves is the occurrence of SVD, which is primarily age dependent. FIGURE 14-2 Aortic valve replacement. FIGURE 14-3 Stented porcine valve. In the 1990s, “stentless” bioprosthetic valves made from porcine aortic valves became available. The advantages of this type of valve versus stented bioprosthetic valves were thought to be: (1) avoiding anticoagulation with a low risk for stroke and (2) improved hemodynamics compared to stented and mechanical valves.5–7 The disadvantages to stentless valves were: (1) more complex operation requiring either a “mini-root” with reimplantation of the coronary ostia ( Figure 14-4) or subcoronary implantation ( Figure 14-5) and (2) data indicating concerns about freedom from SVD. 8 FIGURE 14-4 Stentless porcine root for aortic root replacement. FIGURE 14-5 Stentless porcine root (inset) implanted with a modified subcoronary technique. Some surgeons use stentless porcine valves primarily in patients with BAVs with aneurysms. In this case, the aortic valve, root, and a portion of the tubular ascending aorta are replaced. Current American College of Cardiology/American Heart Association (ACC/AHA) indicate that if a patient with a BAV requires AVR and has an ascending aortic diameter greater than 4.5 cm, then aortic replacement should be undertaken. 9 When this operation is performed with a bioprosthetic valve, current-generation stented valve must be sewn into a separate vascular graft, adding a few minutes to the procedure. With a stentless porcine valve, that step is not required because the porcine valve is packaged as a complete root. Mechanical valves have the advantages of long-term durability and a long track record with designs that have been durable for decades.10,11 The major disadvantages are: (1) the need for lifelong anticoagulation, currently with warfarin, (2) a higher risk of thromboembolism than with bioprosthetic valves, and (3) audible clicking in some patients with several of the mechanical valve types that may be troublesome. A newer model of mechanical valve, the On-X prosthetic heart valve (On-X Life Technologies, Inc., Austin, Texas), first implanted in 1996, has been shown to have low adverse clinical event rates, including 0.6% thromboembolism per patient-year, 0.4% bleeding rate per patient-year, and 0% thrombosis rate when used in the aortic position. 12 An ongoing clinical trial (Prospective Randomized On-X Anticoagulation Clinical Trial [PROACT]) is studying the safety of lower doses of warfarin in patients with high-risk for thromboembolism and antiplatelet drugs only (aspirin/clopidogrel) in patients with low-risk for thromboembolism. 13 The first successful orthotopic placement of an aortic homograft was performed in 1962 by Donald Ross. 14 Much like the procedure for stentless bioprosthetic valves, the operation is more complex than straightforward implantation of a stented tissue valve, because a mini-root may be performed ( Figure 14-6) or the valve may be sewn in the subcoronary position, as with aortic homografts. FIGURE 14-6 Aortic homograft. The perceived advantages to the homograft were: (1) freedom from anticoagulation and a low risk for thromboembolic events typical for bioprosthetic valves, (2) perception that the durability may be higher than that for stented or stentless tissue valves, and (3) belief that homografts are more resistant to reinfection. As to the last advantage, in the setting of endocarditis, most surgeons consider the homograft the valve of choice, although the data for this belief is not very robust. The disadvantages to a homograft are: (1) the increased complexity of implantation, (2) difficulty of reoperation in many patients because of calcification that develops in the wall, 15 and (3) a higher rate of SVD than originally hoped.15,16 Donald Ross also developed the Ross procedure using the pulmonic valve and root autograft with homograft replacement of the patient’s own pulmonic valve ( Figure 14-7). The perceived advantages of this technique were freedom from anticoagulation and decreased risk of stroke. Also, in children, unlike with homografts and bioprosthetic valves, the tissue continues to grow with the patient. The disadvantages are: (1) much more complex operation than other procedures that simply replace the pathologic aortic valve, (2) the potential for dysfunction of two valves, the pulmonic homograft and the autograft, (3) the development of late aneurysms requiring reoperation, and (4) the potential for injury to the first septal perforator when mobilizing the pulmonary autograft. 17 FIGURE 14-7 Ross procedure. Choosing a valve according to the patient’s age is controversial. Findings of two major randomized clinical trials have not been consistent regarding difference in long-term survival between bioprosthetic and mechanical valves.18,19 Although both trials compared first-generation porcine valves and single-tilting disk Bjork-Shiley valves (neither valve is currently in use), the Veterans Affairs Cooperative Study 19 showed improved 15-year survival with mechanical valves than with bioprosthetic valves, but the Edinburgh Heart Valve Trial 18 showed no difference in 20-year survival. Regarding age threshold for valve choice, both the U.S. 9 and European 20 guidelines recommend bioprosthetic valves for patients 65 years and older when only age is considered ( Table 14-1). The European guidelines, however, have a class I recommendation for mechanical valve in patients younger than 40 years and a class IIa recommendation for patients younger than 60 years, recognizing that in patients between 60 and 65 years, other factors impact valve choice. 20 Guidelines for mechanical valves show that requirement for anticoagulation due to a mechanical valve in another position and a condition associated with high risk of thromboembolism are factors favoring the use of mechanical valves ( Table 14-2). On the other hand, contraindication to anticoagulation and planned pregnancy favors the use of bioprosthetic valves ( Table 14-3). Both guidelines acknowledge patient preference after informed consent, balancing the risk of long-term anticoagulation required for mechanical valves and the risk of SVD requiring re-intervention that is associated with bioprosthetic valves. TABLE 14-1 Age Thresholds for Valve Choice *A bioprosthesis is reasonable for aortic valve replacement (AVR) in patients less than 65 years of age who elect to receive this valve for lifestyle considerations after detailed discussions of the risks of anticoagulation versus the likelihood that a second AVR may be necessary in the future. TABLE 14-2 Guidelines Favoring Mechanical Valves TABLE 14-3 Guidelines Favoring Bioprosthetic Valves Long-term follow-up of commonly used bioprosthetic valves for the aortic position show good durability to more than 15 years in several large series ( Table 14-4). Freedom from SVD for stented bovine pericardial valves has been reported to be 82.3% at 15 years for the Carpentier-Edwards bovine pericardial valve (Edwards Lifesciences Corporation, Irvine, California) 21 and 62.3% at 20 years for the Mitroflow aortic pericardial heart valve (Sorin Group, Milan). 22 For stented porcine valves, the freedom from SVD has been reported to be 63.4% at 20 years for the Hancock II valve (Medtronic, Inc., Minneapolis, Minnesota) 23 and the freedom from reoperation for SVD to be 61.1% for the Biocor valve (St. Jude Medical, Inc., St. Paul, Minnesota). 24 All of these series stratified their results by patient age, which is the major determinant of durability, and found that 20-year freedom from reoperation for SVD in patients 70 years and older to be between 84.8% (Mitroflow) 22 and 100% (Hancock II). 25 TABLE 14-4 Structural Valve Deterioration of Bioprosthetic Valves AVR, Aortic valve replacement; CI, 95% confidence interval; MVR, mitral valve replacement. Controversy exists for valve choice in younger patients who would like to avoid the risk of complications associated with the use of long-term warfarin therapy required for mechanical valves. Even in patients as young as 45 years, freedom from SVD was about 85% at 10 years and 55% at 15 years with the Carpentier-Edwards pericardial valve in a study from the Cleveland Clinic. 26 Also in a review of very young patients (mean age 22.7 ± 6.8 yrs), freedom from all bioprosthetic valve-related complications was 85.8% at 8 years. 27 El Oakley et al 28 calculated that for a 50-year-old patient, the risk of valve-related morbidity over the projected life expectancy of the patient was 108% with a mechanical valve, compared with 48% with a bioprosthesis. Different types of bioprosthetic valves also have been compared, and results may not be uniform. Rahimtoola 29 compared reports of SVD with the use of bovine pericardial valves and porcine valves and found a much lower rate of SVD with pericardial valves. For young patients who eventually develop SVD requiring intervention in the future, repeat AVR may not be mandatory. The possibility of TAVI for failed bioprosthetic valves (valve-in-valve procedure) has the potential of making AVR with a bioprosthetic valve more attractive to the patient (see Chapter 15). The feasibility of the valve-in-valve procedure has been demonstrated in single-center series.30–33 Procedural success was 100% in one series of 23 patients, and another report of 47 patients noted one intraoperative death.30,34 An international registry of 202 patients demonstrated 30-day mortality after valve-in-valve procedure of 8.4% and 1-year survival of 85.8%. 35 Procedural concerns, however, included device malposition in 15.3% of patients, coronary ostial obstruction in 3.5%, and relatively high mean gradients, 15.9 ± 8.6 mm Hg. Aortic valve repair can be performed in selected patients with aortic regurgitation (AR). Unfortunately, valve repair in patients with aortic stenosis (AS) involving leaflet decalcification is not a feasible treatment option and has been associated with early postoperative AR due to leaflet scarring and late restenosis due to recalcification. As with mitral valve repair, the benefit of aortic valve repair over AVR is avoidance of prosthetic valve-related complications such as thromboembolism and infective endocarditis. Data on aortic valve repair durability are limited to experienced centers, but 10-year freedom from reoperation can be as high as 93% in tricuspid aortic valves.36–38 The durability of BAV repair, however, is less than that of tricuspid aortic valves, and repair for these patients with BAV remains controversial. No guidelines exist for aortic valve repair. Repair of a normal, but regurgitant tricuspid aortic valve uses a combination of cusp repair and annuloplasty. Typically, one or more cusps are redundant which causes cusp prolapse. Central free margin plication at the nodulus of Arantius effectively shortens the cusp, resulting in a higher zone of coaptation with the other cusps ( Figure 14-8). 39 An alternative method of cusp shortening is free margin resuspension with a continuous over-and-over suture from commissure to commissure ( Figure 14-9). This technique is also useful for closing cusp fenestrations, which are typically located near the commissures, where cusp stress is highest. The least common technique of cusp repair is cusp extension with pericardium in cases of inadequate cusp tissue ( Figure 14-10). Occasionally, leaflet perforations such as those that occur after healed endocarditis can be simply repaired with a pericardial patch ( Figure 14-11). A reduction annuloplasty may also be required in cases of annuloaortic ectasia. The simplest technique is the commissural plication ( Figure 14-12). This technique achieves narrowing of the interleaflet triangle below the commissure and reduces the diameter of the aortic root, thereby increasing coaptation of the cusp surfaces. Novel techniques utilizing an external aortic annulus ring for the purpose of downsizing the aortic annulus have been described, but these rings are not currently commercially available in the United States. 40 FIGURE 14-8 Free margin plication. FIGURE 14-9 Leaflet shortening. FIGURE 14-10 Leaflet extension. FIGURE 14-11 Repair of healed endocarditis. FIGURE 14-12 Aortic commissuroplasty. Repair of the BAV can be performed with similar techniques of cusp repair and reduction annuloplasty. The goal of BAV repair is to restore a competent BAV rather than to create a tricuspid aortic valve. In cases with equal-size cusps and commissure oriented at 180 degrees to each other, repair can be performed readily as with a tricuspid aortic valve. However, in the more common type of BAV involving a conjoint (fused) cusp ( Figure 14-13), the raphe may be sclerosed and immobile, requiring additional techniques. In these cases, a triangular resection of the raphe can be performed with reapproximation of the edges to create a shortened and pliable cusp ( Figure 14-14). When tissue the conjoint cusp is inadequate, the raphe can be released from the commissure and shaved to improve cusp mobility. Judgment must be used in cases with severely sclerosed valves, because the durability of a repair of a diseased BAV may be less than even that of a bioprosthetic valve. FIGURE 14-13 Bicuspid valve.
Surgical Approach to Diseases of the Aortic Valve and the Aortic Root
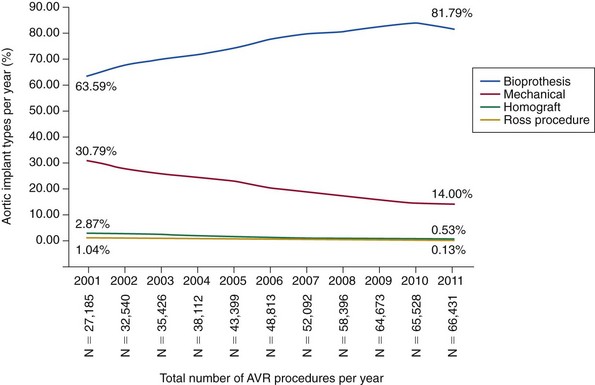
Bioprosthetic valves are most commonly implanted in the current era. Mechanical valves, homografts, and pulmonary autografts are all declining in use over time. AVR, Aortic valve replacement.
Aortic Valve Replacement/Repair
Tissue Valves: Stented
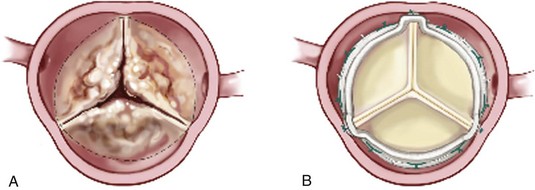
A, Calcified native aortic valve. B, Stented bovine pericardial valve. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
The heterologous porcine tissue leaflets are attached to the supporting frame with an incorporated sewing ring. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208).
Bioprosthetic Valves: Stentless
The porcine root completely replaces the native aortic root, and coronaries are reimplanted. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
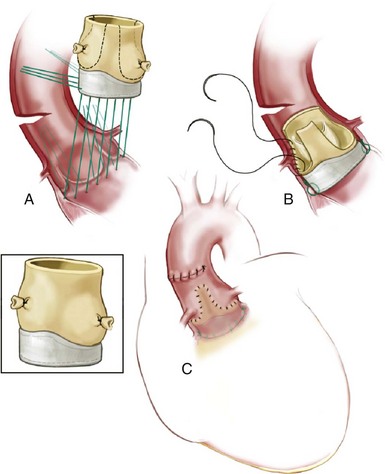
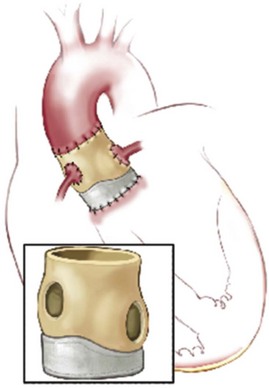
A, Proximal interrupted suture line in a circular plan at or below annulus. B, Distal, continuous polypropylene suture line attaching residual aortic wall to native aortic wall, running below the coronary ostia and preserving the porcine noncoronary sinus. C, Aortotomy closed showing relationship of distal suture line to coronary ostia. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
Mechanical Valves
Aortic Homografts
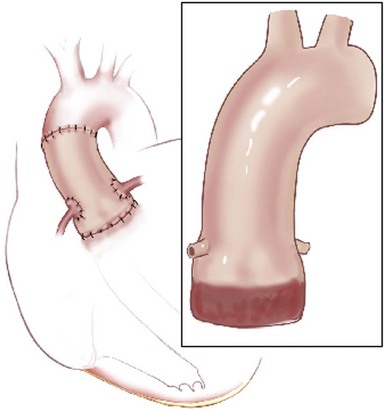
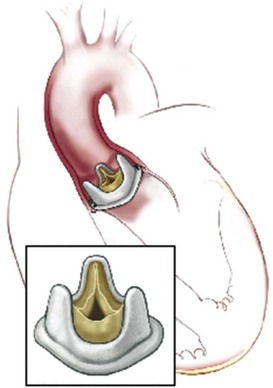
The root replacement configuration using the cryopreserved aortic homograft with reimplantation of coronary ostia is shown. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
Ross Procedure
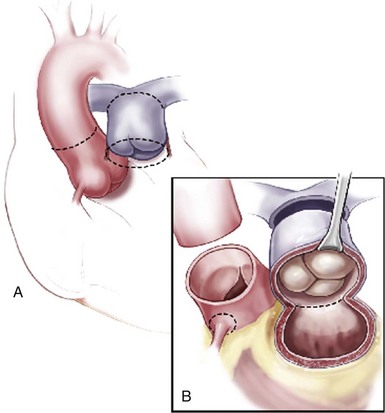
Incision lines are illustrated (dotted lines) for aortic (transverse and distal) and pulmonary roots. The distal pulmonary incision is made first to allow inspection of the valve and to enable accurate placement of the proximal incision below the annulus. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
Guidelines for Valve Choice

ACC/AHA 2006 GUIDELINES
ESC/EACTS 2012 GUIDELINES
Patient already undergoing anticoagulation for mechanical prosthesis in another position
Class IC
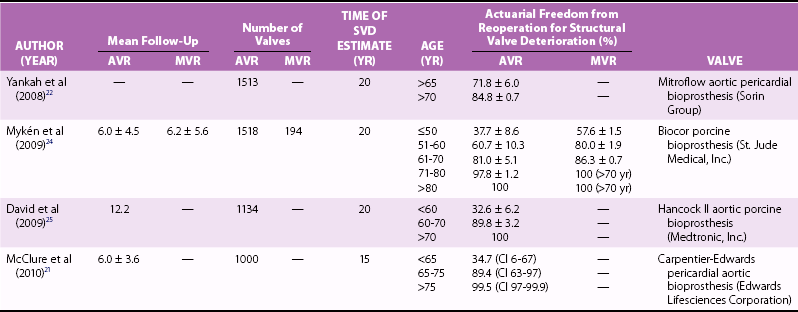
Class IC
Patient preference
Class IIaC
Class IC
Accelerated risk of structural valve deterioration (Age <40 years, hyperparathyroidism)
None
Class IC
Patient already undergoing anticoagulation due to high risk of thromboembolism (atrial fibrillation, venous thromboembolism, thrombophilia, severe left ventricular dysfunction)
Class IIaC
Class IIbC
Reasonable life expectancy (>10 years) and high risk for future “repeat” aortic valve replacement
None
Class IIac
ACC/AHA 2006 GUIDELINES
ESC/EACTS 2012 GUIDELINES
Anticoagulation contraindicated
Class IC
Class IC
Patient preference
Class IIaC
Class IC
Reoperation of mechanical valve thrombosis despite good long-term anticoagulation
None
Class IC
Woman of child-bearing age contemplating pregnancy
Class IIbC
Class IIaC
Low risk for future “repeat” aortic valve replacement
None
Class IIaC
Aortic Valve Repair
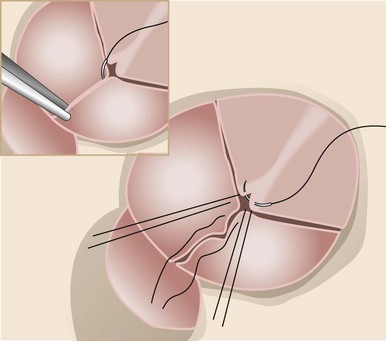
After the prolapsing leaflets are identified, the free margin is plicated at the nodulus of Arantius with a simple, interrupted 5-0 polypropylene stitch. Shortening the free margin brings the leaflet coaptation surface higher in the aortic root. (From Schafers HJ, Langer F, Glombitza P, et al. Aortic valve reconstruction in myxomatous degeneration of aortic valves: are fenestrations a risk factor for repair failure? J Thorac Cardiovasc Surg 2010;139:660–4.)
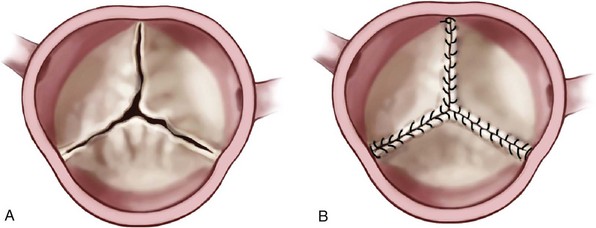
A and B, Continuous over-and-over suture from commissure to commissure is one method of shortening leaflets; 6-0 expanded polytetrafluoroethylene (GORE-TEX) is suggested for this maneuver, with the knots placed outside the aorta. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
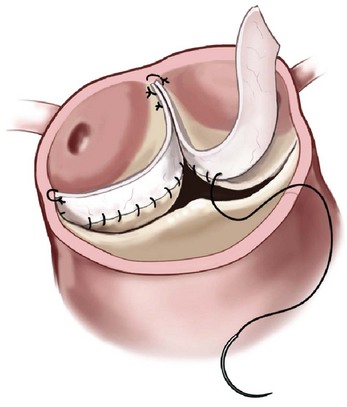
When the aortic valve leaflet is retracted or shortened, the cusp can be extended using pericardium. The pericardium is sewn from commissure to commissure, along the free margin of the leaflet, thereby enlarging the coaptation surface. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
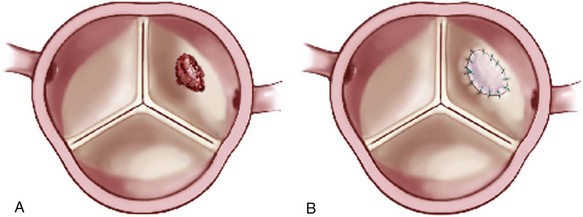
A simple leaflet perforation (A) can be repaired with an autologous pericardial patch (B). (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
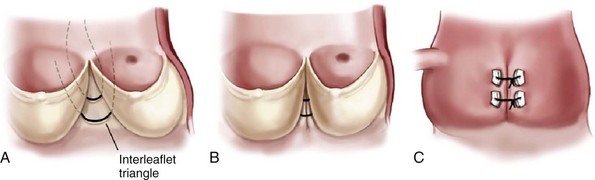
The wide interleaflet triangle (A) is narrowed with sutures that plicate this area to increase coaptation. B, View from inside the aorta; C, External view. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)
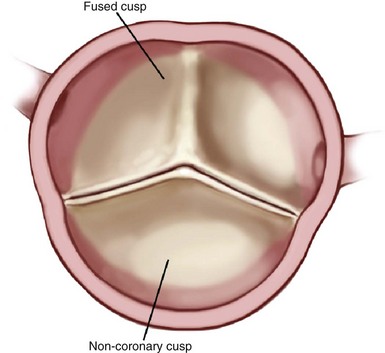
The most common configuration is really a fusion of two leaflets (most often right and left coronary leaflets) with a rudimentary commissure of raphe where the normal commissure would be. (Reprinted from Stelzer P, Adams DH. Surgical approach to aortic valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald’s heart disease. 3rd ed. Philadelphia: Elsevier Science; 2009. p. 187–208.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgical Approach to Diseases of the Aortic Valve and the Aortic Root
Only gold members can continue reading. Log In or Register to continue








